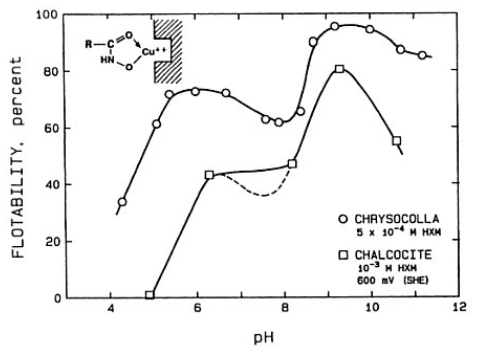
The effect of pH on the flotation response of Chrysocolla and chalcocite with potassium octyl hydroxamate as collector. The potential for the chalcocite system is slightly oxidizing.
A detailed electrochemical investigation of chalcocite with a collector that does not oxidize, namely a chelating compound, potassium octyl hydroxamate shows the pH dependence of the floatability of chrysocolla (a hydrous copper silicate) and that of chalcocite. The flotation response of chrysocolla shows essentially two peaks, the lower one under the conditions where copper ions hydrolyze (about pH 6) and the second where the hydroxamate hydrolyzes to hydroxamic acid (about pH 9). In the case of chalcocite, under reducing potential conditions, there is no flotation of chalcocite with hydroxamate as collector. But under oxidizing conditions, the lattice copper ions become available to chelate with hydroxamate and the behavior is now somewhat similar to that with chrysocolla.
Laboratory experimentation has included not only selective flocculation, but also floc flotation, shear flocculation, and chelating flotation reagents, and the fine-particle investigations continue today.