Table of Contents
The iron-nail method of assaying has been used for a number of years, but has not met with the approval of all assayers. The method possesses advantages which may be given as follows: (1) no preliminary treatment is required; (2) a lead button of proper size can be obtained; (3) it is economical. On the other hand, it has its disadvantages, which may be summed up as follows: (1) it is not applicable to ores containing large amounts of impurities other than sulphur, as practically all the base metals will contaminate the lead button ; (2) the slag is unsatisfactory in the larger number of fusions; (3) the method was known to give low silver results under certain conditions and the gold results were questionable. It was with the idea of determining the extent of this latter disadvantage that the following investigation was undertaken.
It is well known that the fusion obtained in the lead assay is far more satisfactory than that in the “ nails method ” for gold and silver. The principal difference in the charges as a class is the presence of considerable reducing agent in the lead charge, and a limited oxidizing action in the nails charge, due to PbO.
The possibility of improving the nails fusion by simulating the lead charge was the reason for using argol in some tests, even though there were reasons for expecting this would have an objectionable effect on the results.
Preparation of the Gold-Bearing Material
The materials containing gold and silver used in the following experiments were prepared rather than selected, in order to have a definite working product excluding undesirable elements. A high-grade material was made so that any difference in the results obtained from the several methods employed might be more marked.
The material containing the gold was prepared as follows: Some pure quartz was ground to pass 60 mesh, and sized on an 80-mesh screen. Cupels were made of the —60 + 80 material, using sodium silicate (1 of silicate to 6 of water) as a binder. These were very soft and crumbly, so the finer (—80) quartz was tried, with sodium silicate as above. With the finer material the cupels were very firm; they were dried and heated at a red heat for five minutes. Approximately 5 g. of metallic gold was dissolved in aqua regia, heated until all the chlorine was removed, and the solution diluted to 20 cc. The cupels weighed 15 g. apiece, and into each was poured 3 1/3 cc. of gold solution. These were then dried and heated at a red heat to break up the gold chloride and precipitate the gold in the metallic state. At the points of contact of the cupel, due to the unevenness of the base and the asbestos on which they were dried, metallic gold, which was afterwards removed, was seen; otherwise the gold was in a very fine condition, not visible with a magnifying glass. The cupels were ground to pass 120 mesh, and no metallic particles were visible on the screen.
Preparation of Silver Sulphide
Cupels similar to those used to absorb the gold chloride solution were made, and in these were placed 3 1/3 cc. of silver nitrate solution prepared by dissolving 23.8 g. of silver nitrate in 20 cc. of water. The solution was absorbed in about 2 min., but not as quickly as the gold solution. The cupels were placed in a 20 per cent, sodium sulphide solution over night, and then in a 5 per cent, sodium sulphide solution for 5 hours. They were washed to remove the excess of sodium sulphide, and dried, not above a temperature at which they could be handled with the fingers at any time.
The iron pyrite used in the following experiments was ground to pass a 120-mesh screen. It contained 47.4 per cent, of sulphur (= 88.9 per cent, of FeS2) and had a reducing power equal to 10 g. of lead. :
Assay of the Gold- and Silver-Bearing Materials
The assays were made by the crucible method, and a check was also run with each assay. The charge used to assay this material was as follows:

For the checks weighed quantities of gold and silver were taken and added to a charge similar to the above.
The results were as follows:
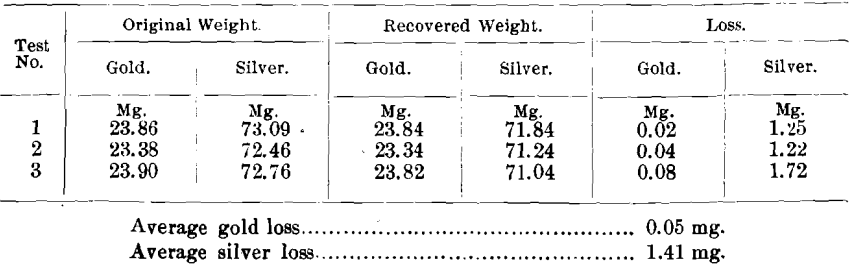
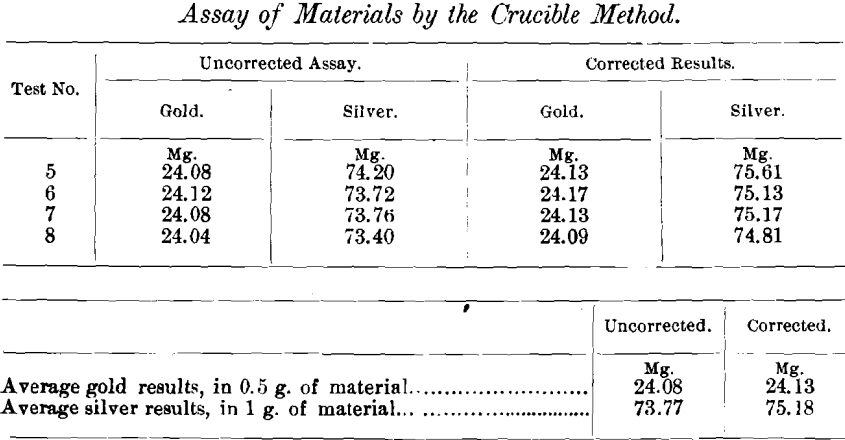
The different sulphur determinations in the slags were made as follows:
Total Sulphur.—With 0.5 g. of finely powdered slag, was mixed 3 g. of zinc oxide and 0.75 g. of dry sodium carbonate. The mixture was heated at a dull red heat for 15 min. After cooling, the mixture, which is easily removed from the crucible if the temperature was not too high, is treated with water and brought to a boil, filtered, the filtrate acidified, boiled, and precipitated with barium chloride.
Sulphide Sulphur.—Was determined by treating the sample with HCl, absorbing the H2S gas in ammoniacal cadmium chloride and titrating with iodine solution, using starch as an indicator.
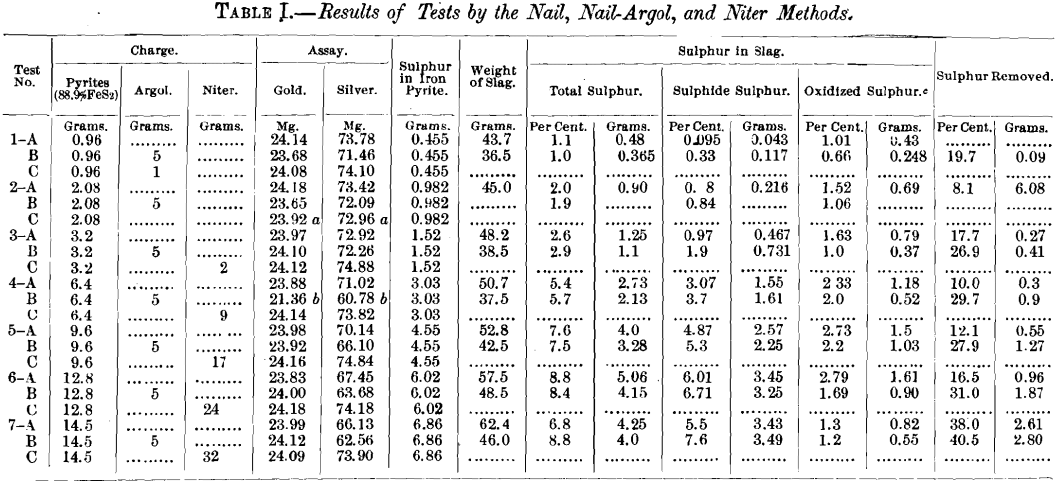
The following experiments were carried on with varying mixtures of pyrite and silica, to which were added definite amounts of the gold and silver material. The mixtures contained 6, 13, 20, 40, 60, 80, and 88.9 per cent, of pure pyrite, or 6.7, 14.6, 22.4, 44.9, 67.4, 90, and 100 per cent, of the impure pyrite (88.9 per cent, pure), enough silica being added to the pyrite to make 0.5 assay ton.
While these tests were made primarily to determine the value of the nails charge, it was thought advisable to run niter fusions on the same series, particularly in view of the oft-repeated statement that charges requiring more than 20 g. of niter were not satisfactory. The nails charges (Tests A and B) were:
Pyrite mixture…………………………………………………0.5 A. T.
Sodium carbonate………………………………………………30 g.
Litharge…………………………………………………………….25 g.
Borax glass………………………………………………………..10 g.
Nails, four…………………………………………………………10 d.
Test A contained no argol, while Test B contained 5 g. in each assay.
For the niter tests (Test C) the following charge was used :
Pyrite mixture……………………………………………..0.5 A. T.
Sodium carbonate…………………………………………….15 g.
Litharge…………………………………………………………105 g.
Silica………………………………………………………………..5 g.
Borax glass……………………………………………………….7 g.
Niter or argol to give the required amount of lead.
The silica and so large an amount of flux is not required in the lower pyrite mixtures, but was used to make the flux charge uniform. The number of grams of pyrite in 0.5 assay ton mixture is indicated in Table I.
The fusion of the nails charges which contained no argol was of the usual character. Those containing small amounts of pyrite were fairly satisfactory, but as the percentage of pyrite increased there was less complete dissolution of constituents; the nails were badly corroded, and in some cases to such an extent that they sank beneath the slag. The lead did not collect perfectly in all cases, leaving small particles in the crucible on pouring.
The charges containing argol were much better, but showed the same general effect with the increased amount of pyrite. Complete dissolution seemed to take place, and in no case were the nails corroded to such an extent that they disappeared. The charges poured much better, showing a good collection of the lead. This improved condition is unquestionably due to the decrease in oxidation of constituents in the charge. As this charge is highly basic, containing
Uncorrected results of gold material…………………………………………24.08 mg.
Uncorrected results of silver material………………………………………..73.77 mg.
large amounts of sodium carbonate, the sulphides are readily soluble; but if the iron present is converted to oxide this will not be readily taken into solution. It is not permissible to make this slag highly acid to take these oxides into solution, as the sulphides would then fail to dissolve.
It was noticed in the different fusions that the character of the furnace atmosphere, whether oxidizing or reducing, had considerable effect on the charge. If the atmosphere was strongly oxidizing the fusion was not nearly as good as when it was reducing, this apparently being due to the same causes as where argol is present in one case and absent in the other.
The largely increased weight of slag in charges high in pyrite is due partly to the greater amount of iron dissolved, and partly to the material corroded from the crucible on account of the much more basic character of these slags.
From the results, it will be noted that the silver losses increase with the sulphur content. Also, that in charges containing argol the loss is somewhat greater than where the addition of argol was omitted. The principal difference in the slags of these two methods, which could affect the results, is in the percentage of sulphide sulphur present, the charges containing argol having a much greater percentage. This indicates that the silver losses are due to sulphide sulphur.
This explanation seems quite reasonable, as these slags are somewhat of the nature of a matte, particularly where sulphide sulphur is high, and it is well known that mattes are good solvents for silver.
For the same reasons, it was expected that the gold loss would also increase to a considerable extent. It will be noted from the table that the increased loss is not proportionate to the silver, nor does it seem to increase progressively with the sulphur.
In a number of the experiments, the nails were weighed before and after fusion of the charge. It was seen that considerably more iron was removed from the nails than was necessary to form iron sulphide; also, on examining the nails, that they were covered with a dull brown coating. The following figures show the amounts of iron removed from the nails. Tests Nos. 8 and 10 were nail assays; and, to Tests Nos. 9 and 11, 5 g. of argol were added.
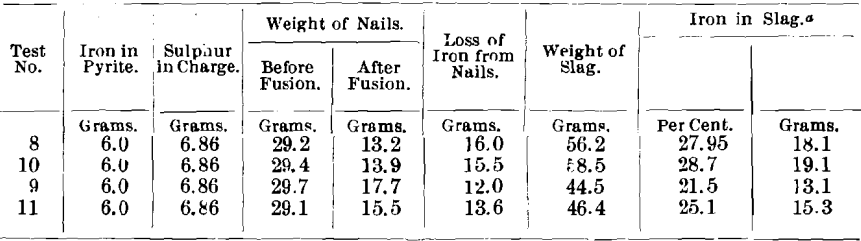
Some of the coating or scale on the nails was removed and analyzed for total sulphur, sulphide sulphur, and iron. The results were as follows:

The scale was considered to be iron sulphide, but this was impossible from the small percentage of sulphur. A portion of the scale from Nos. 10 A and 10 B was ground and did not show any metallic particles. Further, a portion of this scale was magnetic, and the magnetic portion from No. 10 A contained 59.4 per cent, of iron, and the magnetic portion from No. 10 B contained 59.35 per cent, of iron.
The non-magnetic portion from Nos. 10 A and 10 B became magnetic when heated in the reducing flame of a blowpipe, which indicates the presence of ferric oxide (Fe2O3). The literature gives this as iron sulphide (FeS), but indications are to the contrary, and the point was thought worthy of further investigation, therefore the following tests were made:
Fusion (Test No. 12) was made, using this charge:

Also, fusion (Test No. 13), using the same charge, except that no sodium sulphate was added.
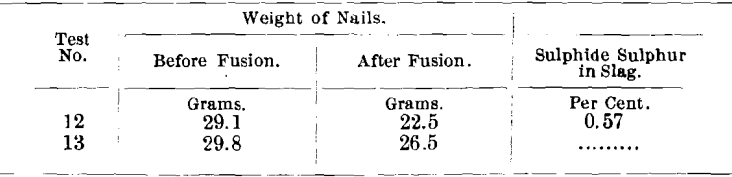
In fusion No. 12, the nails were corroded badly beneath the slag and also the portion above the slag. The scale on the nails (from the part beneath the slag as well as the part above it) was found to contain a magnetic portion, as before, and analyzed 52 per cent, and 71.4 per cent, of iron, respectively.
In Test No. 13, there was also a scale on the nails above the slag, but the portion of the nail in the slag was bright and not corroded.
This scale, as before, contained a magnetic portion, which analyzed 68.6 per cent, of iron.
These results clearly indicate that metallic iron under above conditions reacts with sodium sulphate (Na2SO4), producing sulphide sulphur and iron oxide, and explain the excessive corrosion of nails during long fusion in an oxidizing atmosphere. Apparently, there is a cycle in the oxidation of sulphide sulphur to sulphate by the air, reduction of sulphate to sulphide by the iron, and reoxidation by the air. The presence of an organic reducing agent interferes with this oxidation, which is the reason for less corrosion of the nails in charges using argol. The production of this large amount of iron oxide accounts for the unsatisfactory nature of the slag, as the slag is not of a character suitable to dissolve quantities of this material, which remains more or less in suspension. Further, it is responsible for rapid attacking of the crucible, which is composed of constituents that are not conducive to a satisfactory slag with the fluxes employed.
Conclusions
- The nails method gives low results for silver with ores containing more than certain amounts of sulphide sulphur, this loss increasing with the percentage of sulphur in the ore, reaching a maximum of about 10 per cent, in the straight nails charge under the conditions of this test. In the case where argol was used, giving increased sulphide sulphur in the slag, the maximum loss was about 15 per cent.
- The gold results are irregular and somewhat lower, possibly 1 per cent. The loss does not appear to be affected by the addition of argol to the charge.
- The method should not be used for silver determinations in high sulphide ores, except for approximate results. When the sulphur in the ore is not much greater than will be oxidized by the PbO, a fair approximation may be obtained. The method will give fairly good gold results on medium- and low-grade gold ores, say one ounce, as the loss is less than the accuracy of ordinary work.
- The niter method gives good results even with as much as 30 g. of niter present in the charge and a 30-g. button is necessary in assays containing a large amount of niter.
- The portion of the iron nails which projects into the lead is converted to sulphide, but the greater amount of iron, that in contact with the slag, is removed as iron oxide.
- The scale on the iron nails is not iron sulphide, but a mixture of ferric oxide, magnetite, and slag.
- To obtain a good slag by the nails method with slight corrosion of nails, it is necessary to protect the fusion from oxidation. This, however, will lower the silver results.
Discussion
A. M. Smoot, New York, N. Y. (communication to the Secretary):—The nails method is given in many text books on assaying as a standard method for gold or for gold and silver in ores. It appears to be taught in the laboratories of technical and scientific schools as a standard method, and to some extent it is used by assayers in actual practice, although the class of ores to which it may be applied is limited to those free from reducible metallic compounds, the metals of which would enter the lead button.
The experiments of Messrs. Hall and Drury show the inaccuracy of this method for silver, in heavy sulphides, but not so clearly the shortcomings with regard to gold. It seems certain that low silver is caused by the solubility of silver in iron-alkali sulphide as compared with its solubility in molten lead; that is, silver is distributed between iron-alkali sulphide and metal in the charge in proportion to solubility at the particular temperature of the fusion. Lead is a fixed quantity in these assay charges, but iron-alkali sulphide increases with increased sulphur, so the more sulphur in the charge, the lower the silver results.
Considering tests 1A and 2A, in Table I, it will be seen that the amount of pyrite in these charges was so small as to be fully oxidized by the litharge; consequently, the only sulphide sulphur likely to be in the slag would be produced from the reducing action of iron on sodium sulphate. Analyses of the slag show the sulphide sulphur to be very small, practically negligible in these charges. Both silver and gold results are substantially correct, gold being fully as high as in any of the niter-excess litharge charges (tests C), and silver, although a little lower, yet within the limits of ordinary assay errors. When sulphur in the charge increases beyond the amount which can be oxidized by 25 g. of litharge the silver figures drop proportionally to the increase in sulphur. It is quite evident that no iron at all was necessary in 2A, and that in 1A it was only necessary as a reducing agent to produce a proper sized lead button. In this charge a little carbonaceous reducing agent—say a gram of argol—could advantageously have been substituted for nails. The 1A and 2A charges are substantially excess-litharge charges, the slags being of the order of bisilicates. In all the other A charges, 3, 4, 5, 6, and 7, wherein pyrite exceeds the amount that can be oxidized by 25 g. of litharge, silver is markedly and increasingly low as the sulphur increases, and gold is also low, but by a practically fixed amount, averaging, say, 0.20 mg.
In the B experiments, wherein a large excess of argol was used, the silver results are of the same order as in the A experiments, going uniformly lower as the sulphide sulphur increases. The gold results, however, are different; charges 1B and 2B, containing only small amounts of pyrite, yield markedly low gold, whereas, omitting 4B, from which some lead was lost, the other B charges are only slightly low. It seems that, unlike silver, gold is not soluble in alkali-iron sulphide. A reasonable explanation for the low gold in 1B and 2B might be found in the nature of the slag. This is nearer a bisilicate than a monosilicate because of the large amount of silica added to make the weight of the “ ore ” equal to 0.5 A. T. Thus in 1A and 1B, 13.5 g. of silica were added; in 2A and 2B, 12.5 g. In these charges there is, therefore, no excess of soda to form iron-alkali sulphides, but as the pyrite is increased and the silica correspondingly decreased the charges become more basic, with higher results in gold in the case of the B experiments. It should be kept in mind that in the A charges no extra reducing agent is present, the slag is unsatisfactory, and the ore is probably not fully decomposed, while in the B charges argol is present in large excess. Probably in the B charges the litharge is reduced early in the fusion period, whereas in the A charges it is more slowly reduced by the sulphur in the ore. It is reasonable to suppose that the sulphide formed in 1B and 2B is really present as an iron matte disseminated through the slag, but not dissolved in the form of an iron-alkali sulphide, because there is no excess of alkali in these charges to form such a compound. As iron matte is an excellent solvent for gold, low results may be expected where it is formed.
In the case of the other B experiments, Nos. 3, 5, 6, and 7, where sulphur increases and silica decreases, presumably with the formation of alkali-iron sulphide in the slag, the gold figures are considerably higher than in the A experiments, subsequent to 3A.
This argument, based on Hall and Drury’s experiments, points to the conclusion that the nails method would be improved for gold by increasing the alkali, so as always to form alkali-iron sulphide instead of iron matte, as well as by adding an excess of an organic reducing agent, thereby producing a better slag. Experimental evidence in support of this suggestion is at present slender, but so far as it goes it supports the theory.
Two samples of heavy sulphide (pyritic) ores, assayed in Ledoux & Co.’s laboratory by the excess-litharge method, gave the following in ounces of gold per ton :

The same sample by the nails method, using the conventional charge of 0.5 A. T. ore, 30 g. sodium carbonate, 10 g. borax glass, 25 g. litharge, and 4 nails, gave:

These results fully confirm prior experience, that the nails method, as ordinarily conducted, gives low gold. The nails method, modified by adding 5 g. of argol to the preceding charge and increasing the sodium carbonate to 60 g., gave :

It will be seen from the above that while the usual “ nails ” charges give markedly low results, the modified “ nails ” charges are commensurate with the excess-litharge method.
W. J. Sharwood, Lead, S. D. (communication to the Secretary): —It seems possible that one of the principal differences between nail-method slags and the slags formed in the ordinary lead assay, in addition to those mentioned by the authors, and perhaps the chief cause of the less satisfactory character of the former, may be the presence of a larger proportion of ferrous sulphide, or, as usually stated, of ferrous alkaline sulphide. This is due to the relative smallness of the usual lead charge and its commonly lower proportion of sulphur.
Thus, with the ordinary 5-g. lead assay charge, a sample of pure galena would carry only 0.67 g. of total sulphur. With an ore consisting of one-third each of galena, blende, and pyrite, there would be 1.66 g. of sulphur. The proportion of alkaline flux per unit of ore, calculated to Na2O and K2O, is commonly somewhat greater in the lead assay than in the mixture used by the authors, and the large ratio of potash to soda also makes it decidedly more fusible than an unmixed soda flux.
The valuable data in Table I confirm what is, I believe, the general experience of assayers, that the nail method tends to give decidedly low silver results, but is reasonably accurate in regard to gold; while the niter fusion, if carefully performed, is fairly satisfactory for both gold and silver. The silver losses reported in this table for the nail method seem, however, unusually large.
Thus, in the four most highly sulphuretted charges (40 to 90 per cent, pure pyrite) the silver results, using nails and argol together, average 14 per cent, below the truth. Without argol the two highest in pyrite give errors of nearly 10 per cent., while with 40 and 60 per cent, of pyrite the silver is about 4 per cent, too low. On the other hand, the gold tends to be slightly higher with increasing pyrite—a result which might be modified were a large number of determinations made and averaged. The average gold value given in the table for the nail method is very nearly that of the uncorrected assay without pyrite. Is it possible that there is a trace of gold in the pyrite used ?
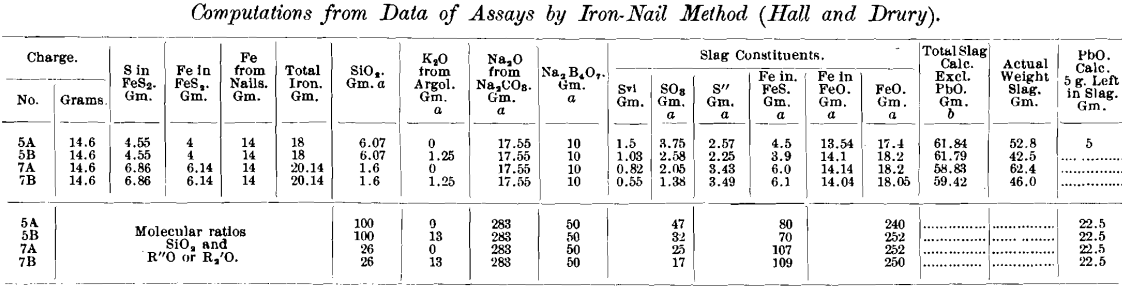
The authors unfortunately do not mention the amounts of iron and lead in the slags, nor the size of the lead button obtained; presumably nearly all the lead was reduced. In the accompanying table I have taken the liberty of assuming, as an approximation based on the authors’ data (p. 37), that 14 g. of iron was removed from the nails in each of the tests numbered 5 and 7, and that 5 g. of lead remained in the slag. The tabulated molecular ratios indicate the nature of the slags obtained, from a chemical standpoint, perhaps more clearly than the mere percentage analysis. Even if as much as 5 g. of lead remains in the slag, it is evidently of relatively small importance compared with the other constituents, mostly of low molecular weight.
In some assays made several years ago, with charges of 0.5 and 1 assay ton of Frue concentrates containing up to 50 per cent, of pyrite and pyrrhotite, using nails or iron filings, a few slags were analyzed, and it was found that nearly half the sulphur remained in the slag as sulphide. It is interesting to note that the same is true of the high-pyrite charges tabulated in this paper.
The size of the nails employed in these experiments is not stated. This seems to be a matter of some importance. Frequently a 20d. (4-in.) nail is used, either wire or cut. The late Richard Smith, when teaching assaying at the Royal School of Mines, used to recommend the cut nails then in vogue, used head downward to expose the greatest surface possible; alternatives being lengths of nail rod or pieces of 0.5-in. hoop iron, sometimes bent U-shape; or substituting a suitable amount of iron filings or turnings.
The manipulation necessary when large nails or long pieces of metal are used and removed, is a time-consuming inconvenience, while the waste of metal is considerable, as it is rarely safe to use a nail more than once. It seems desirable, therefore, to use iron in some form exposing more surface, adjusting the amount to the requirements of the case, so that all may be consumed, leaving no excess to be removed.
The idea is not a new one. In the case of the sulphide ores of lead, which are partly decomposable by sodium carbonate alone, partly by iron alone, but completely by both together, the use of iron filings is mentioned by Mitchell and by Percy, both of whom refer to still earlier work. In fact, this seems to have been one of the earliest forms in which iron was used for assay purposes. In Cramer’s work on assaying (Mortimer’s translation, 2d ed., 1764) one section is devoted to “ Precipitation by Iron and Lead of Silver out of a Mixture containing a great Deal of Sulphur,” in which the reader is cautioned “You must not use Filings quite spoiled with Rust: For they have no Virtue for absorbing the Sulphur.”
At the Homestake assay office the practice, introduced about 10 years ago, is to use small steel wire nails, a number of which, suited to the material to be assayed, are stuck in the top of the charge before adding the borax cover. A size found well adapted to charges of tailing carrying from 3 to 5 per cent, of sulphur is a 7/8-in. brad of 18-gauge wire, the diameter of which is about 0.045 in. An old Californian book — Barstow’s Sulphurets, I believe — recommended small tacks for this purpose.
The authors evidently realize the advantage of using normal sodium carbonate (soda ash) as a flux, rather than the bicarbonate to which, for some unexplained reason, so many assayers still adhere. It may be worth while to point out here that soda ash usually costs less per pound, and that a pound of it contains over 50 per cent, more actual fluxing material than a pound of bicarbonate, though the latter gives off about 90 per cent, more gas per pound before action ceases. For every gram of Na2O available as flux, one must use 1.7 g. dry sodium
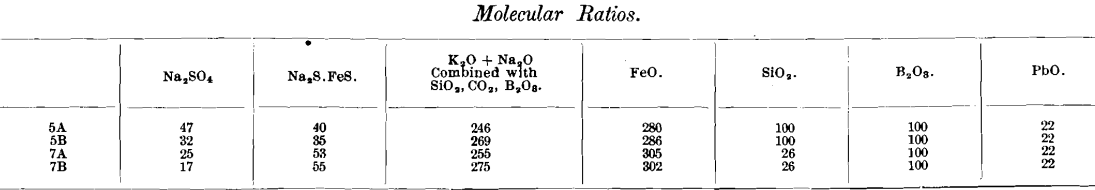
carbonate (soda ash) or 2.7 g. of bicarbonate. If both are pure the volume of gas given off by the soda ash is just one-third of that evolved by an equivalent (not equal) weight of bicarbonate. Thus the amounts finally given off per gram of Na2O available are nearly 500 and 1,500 cc. respectively if measured at 100° C., or about double these volumes at 400° C.
The authors’ data indicate the superiority of the niter assay to the nail method for high-sulphide ores, when silver is of importance. Some assayers, however, avoid large proportions of niter, on account of its tendency to boil over. Others do not hesitate to use 35 g. or more per charge, apparently without disastrous effects. This seems to be one of the instances where experience and close attention to details enable the successful use of a method which presents considerable difficulty to a novice.
A reagent which presents some advantages, and was recommended by Percy but is now little used, is red lead as a substitute for litharge. It contains at least 25 per cent, more oxygen, retaining it up to a fairly high temperature, so that the extra oxygen is available for the oxidation of sulphur or other reducing agents. Some of the redder samples of litharge in the market contain an appreciable amount of extra oxygen.
While it would not be permissible to make the slag highly acid in carrying out the nail assay, it seems desirable to add some silica whenever the percentage of pyrite is high, if only to check the corrosion of the crucibles. I believe this is common practice. One writer on assaying (W. L. Brown) has gone to the extreme of recommending the addition of silica to all assay charges—even with quartzose ores.
L. S. Austin, Salt Lake City, Utah (communication to the Secretary):—Messrs. Hall and Drury have investigated the limitations of the nail assay under certain specified conditions, but one need not accordingly infer that it is to be set aside. It remains a quick and satisfactory method if judiciously used.
The nail assay has been long since tested out in practice, the results obtained by it having been compared with those from scorification and niter-fusion. This has been within the experience of many assayers, who, as the result of their long-continued experiences, have felt that they know how and when to use it.
The prospector frequently brings to the custom assayer samples of ore for assay, sometimes a single piece, sometimes an approximate sample of a face of ore, sometimes a “ grab ” sample, this latter a handful of ore taken at random from a heap, and mistakenly assumed by the taker to represent the heap. Under such circumstances a result a little low, as, for example, an ounce in twenty-five of silver, would be on the safe side, and would make no practical difference to the prospector. For the assay he wishes to pay, or can afford to pay, very little.
Again, a mine assayer may have dozens of assays to make daily, results from faces of stopes, grab samples from ore piles, from ore passing through the receiving bins and from other places, where approximations would serve every purpose.
In the paper several reasons are given in favor of the nail assay and these should have weight. These are: (1) That no preliminary treatment is needed, as in the niter assay. By this I understand the preliminary assay for reducing power, though I find that the skilled assayer, by doing a little panning of the ore or simply by inspection, can, four times out of five, determine the niter which should be added. The object of the preliminary work is of course (2) to insure a lead button for cupelling of the proper size. As to the third point, the method is economical. If the economy is in saving supplies, this should not influence one, but if time is economized, then such economy is another and an important consideration.
Certainly, as stated, the nail assay is hardly suited to ores containing appreciable quantities of impurities: viz., copper, arsenic, antimony, or tellurium. Such an ore would yield a hard button and this would have to be scorified, thus introducing double work, which it is the object of the nail assay to avoid. While this might indeed happen even to an experienced assayer, still it need seldom occur, and he could well afford to take the risk.
If the charges are properly made up, I am hardly prepared to agree with the authors that the slags are unsatisfactory in the larger number of fusions. As to low results with silver, we know that results are lower than by the niter assay, and when ore lots are assayed this latter method is indicated. In such cases, however, the assayer must use his best efforts, and spare neither time nor pains to insure the proper result. One experienced assayer, using the nail assay, asserts that the difference between it and the niter assay should not exceed 0.5 per cent.
The scorification method, formerly so much used, appears of late years to have dropped more out of sight. For all that, it is a most excellent method, and as a control on the crucible assay it may be used to great advantage, especially in the case of impure ores.
Since the nail assay is so convenient, so simple, and so inexpensive, why not use it, preparing for it a charge which can be rapidly measured out ? Such a charge might be thus specified :
Ore ………………………………………………………….. 0.5 assay ton.
Soda…………………………………………………………. 25 to 35 g.
Litharge……………………………………………………. 20 g. or less.
Argol……………………………………………………………. 3 to 4 g.
Borax glass………………………………………………….. 5 to 15 g.
Quartz sand………………………………………………… 0 to 10 g.
Ten-penny nails………………………………………………. 1 to 6.
Considering these fluxes in order:
For most ores 25 g. of soda is enough, but if the ore is nearly all silica 85 g. is needed. When the ore contains but little silica the deficiency is made up by the addition of sand up to the amount of 10 g., knowing that any shortage will be made up from the crucible itself. Glass may be used in place of sand, but to twice the quantity of the latter. I may say that for rather siliceous ores the crucible corrosion is not serious, but one is naturally desirous of saving the crucible for other assays, and here again one must be cautious never to use it again on ores or ore lots when the results must be exact.
Twenty grams of litharge, which will be entirely reduced by the argols, gives a suitable-sized button, and it is not intended that any lead shall be left in the slag. In case the ore contains lead then the litharge is lessened to insure 18 g. of a button.
The quantity of argol is so small that its presence hardly affects the slag. Added to insure the reduction of all the lead in an oxidized ore, it is left standing even when not needed.
One can hardly use less than 5 g. of borax glass and be sure that the top of the charge is covered to exclude the air; 15 g. is added where the ore is decidedly basic.
The nails will vary, according to the judgment of the assayer. Though in an apparently oxidized ore the nails could be omitted, still one is retained as a precaution.
I will give now some examples of fluxing, applying the above principles, and using 0.5 assay ton of ore.
- A quite siliceous ore with little base: Soda, 25 g.; litharge, 20 g.; argol, 3 g.; borax glass, 5 g.; and one nail.
- An ore largely galena: Soda, 25 g.; litharge, 10 g.; argol, 3 g.; borax glass, 5 g.; and two nails.
- A basic, oxidized ore with little or no lead: Soda, 25 g.; litharge, 20 g.; argol, 4 g.; borax glass, 15 g.; sand, 10 g.; and one nail.
- A basic, blendy ore having little lead: Soda, 25 g.; litharge, 20 g.; argol, 3 g.; borax glass, 15 g.; with three nails.
- An ore high in pyrite and having little silica: Soda, 25 g.; litharge, 20 g.; argol, 3 g.; borax glass, 15 g.; sand, 10 g.; and six nails.
Where the work is run hot, with pieces of coke placed at the mouth of the muffle, one should have but little trouble from drops of lead sticking to the nails, and with but little oxidizing effect upon the charge.
In conclusion, I would say that I consider the nail assay to be a speedy and practical method of determining ores, especially those where fairly approximate results will serve; but for obtaining the best and most accurate returns, one should use the niter assay or scorification.
