Table of Contents
A laboratory mineral-dressing investigation was conducted by the Federal Bureau of Mines on four limonitic and four sideritic iron ores from the North Basin of the east Texas iron-ore district. The samples, composited from drill cores, were considered representative of the iron-ore reserves of the area. The objectives of this research were twofold: (1) To devise a mineral-dressing method whereby the yield from currently mined east Texas iron ores might be increased and the grade improved, and (2) to extend such treatment to include the lower grade ferruginous materials that are not amenable to beneficiation by simple washing and, therefore, are not being exploited. Of the mineral-dressing methods used in this investigation, magnetic separation of roasted ore was shown to be the most effective. Results show that, by this method, a laboratory recovery of 82 percent of the iron is attainable at a grade surpassing that obtained by current plant practice and that both limonite and siderite are amenable to concentration by the process.
The existence of large deposits of iron ore in eastern Texas has been recognized for over 100 years. These ores were first exploited during the Civil War and were mined intermittently until about 1911. From that time until 1944 when the Defense Plant Corporation financed the erection of three blast furnaces to use these east Texas ores, production was almost at a standstill.
The two major producers in the area operate ore-washing plants, since most of the east Texas iron ores are not direct-shipping grade.
The Sheffield Steel Co. concentrator uses a comparatively high-grade feed, containing 35 percent or more of Fe, and produces concentrates averaging more than 44 percent Fe by a comparatively simple washing operation.
The Lone Star Steel Co. concentrator feed averages about 23 percent Fe. This feed is one of the lowest grade iron ores currently being used in this country. The process, in brief, involves stage crushing, with intervening scrubbing and sizing to remove fine gangue and recover coarse iron minerals.
The final concentrates are reported to average 44 percent Fe and 22 percent acid insoluble material and to represent a recovery of 48 percent of the total iron in the ore.
The low recovery obtained by the commercial concentrators, together with the large reserves of low-grade ore in the east Texas field, prompted the Bureau of Mines to undertake research to develop methods for increasing the recovery of iron from the ores of plant-feed grade and for extending the methods to those ores not amenable to existing plant practice.
The first phase of this research was devoted to the brown (limonite) ores, and results showed that laboratory recoveries of over 80 percent of the iron were possible.
The research presented in this report, which is a continuation of the preliminary study, is concerned with limonitic and sideritic ores individually and with composites of the two.
Location and Description of Samples
In the North Basin area of the east Texas iron-ore district, the ore occurs principally as horizontal ledges and lenses in weathered greensand. The oxide zone is 45 feet thick or more, while the carbonate zone is as much as 65 feet. Recent figures released by the Bureau of Mines show a reserve of 160 million long dry tons of ore in the North Basin that could be washed to a grade of 40 percent Fe.
To obtain the large representative ore samples required for this investigation, the Bureau of Mines drilled 56 holes 7½ inches in diameter on 4 properties in the North Basin. These properties are held by the Lone Star Steel Co. through either warranty or iron-ore deeds and consist of the Connors, Pouns, and Lattimer properties in eastern Morris County and the Pynson property in western Cass County. The total footage drilled amounted to 2,145 feet, of which 840 feet was in brown iron ore, 806 feet in carbonate ore, and 499 feet in waste. Selected drill cuttings were combined for metallurgical samples.
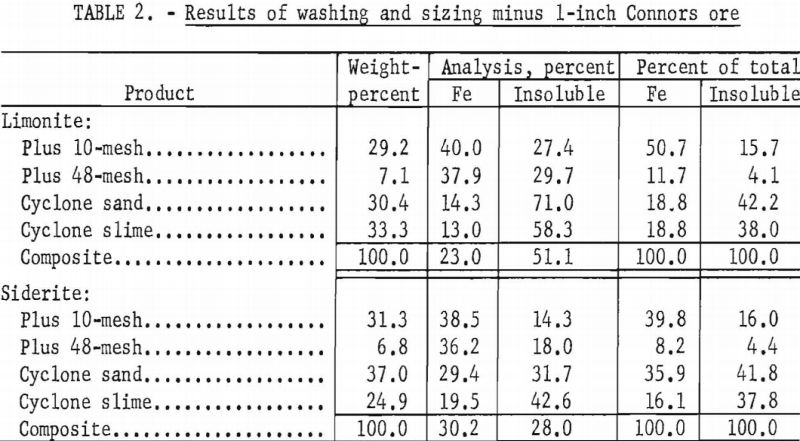
The samples consisted of brown iron ore (limonite) and carbonate ore (siderite). The limonite was composed of both amorphous and crystalline oxides; the siderite was dense and crystalline. Principal impurities were siliceous, free silica as quartz sand, siliceous clay, and residual grains of the hydrous silicate, glauconite. Partial chemical analyses of the individual ores are given in table 1.
Laboratory Beneficiation
Washing and Sizing
Previous work by the Bureau of Mines showed that limonitic ores from the North Basin could be upgraded to an acceptable, but low-grade, concentrate by a simple washing-sizing treatment. Current plant practice in the east Texas field results in a grade concentrate similar to that obtained by washing and sizing the limonitic and sideritic ores investigated. For this study, all samples were crushed to minus 1-inch and blunged at 50 percent solids for 1 hour in a 12-inch-diameter mill equipped with four equispaced lifters. The iron minerals in the scrubbed ores were separated by screening on 10-mesh and 48-mesh sieves; the minus 48-mesh fraction was further classified in a hydrocyclone.
Comparative test runs of blunging in water only and blunging in water containing 1 pound per ton each of sodium silicate and sodium hydroxide showed almost identical recoveries and grade of concentrate. Therefore, the results of blunging with water containing sodium silicate and sodium hydroxide are not tabulated in this report.
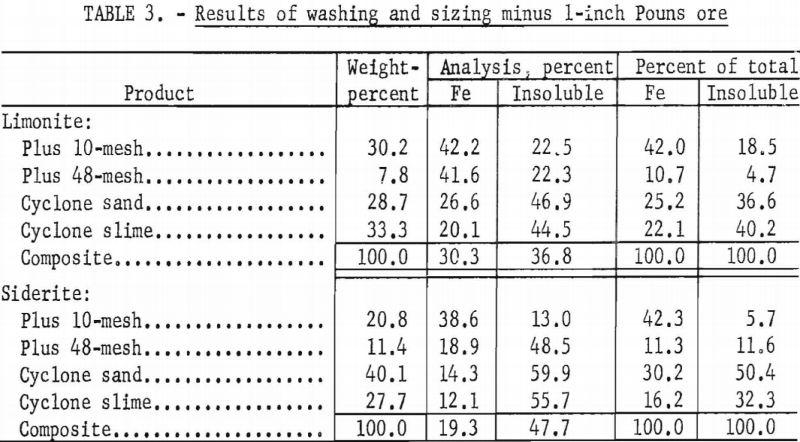
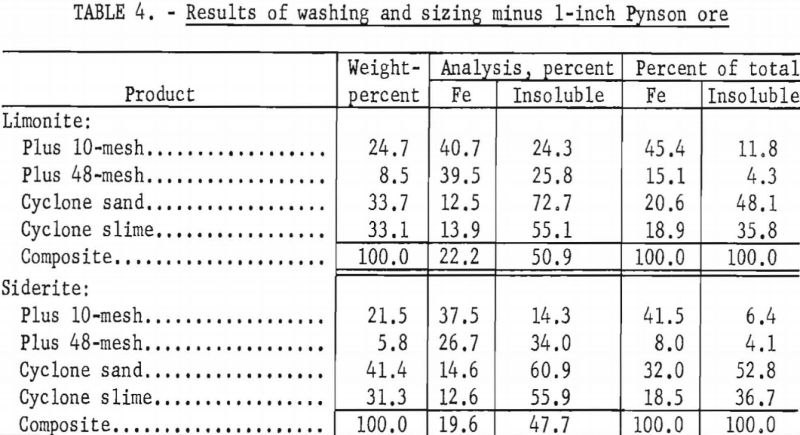
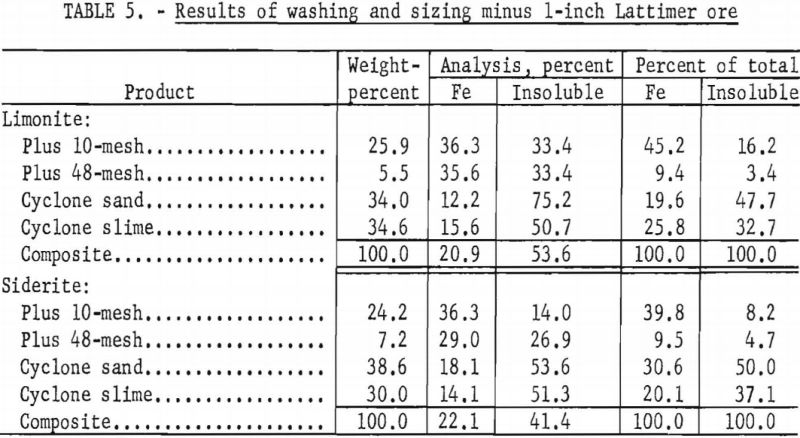
With the laboratory washing-sizing process, limonite ores from the Connors, Pouns, and Pynson properties yielded concentrates averaging 41.0 percent Fe and 24.7 percent acid insoluble material and representing a recovery as high as 46.0 percent of the total iron (tables 2, 3, and 4). The overall concentrate grade and iron recovery approached commercial plant results in the east Texas field. Experiments on the Lattimer limonite (table 5) failed to produce the 40 percent iron concentrate that was considered the minimum acceptable grade. The siderite ores also failed to yield a 40 percent iron concentrate (tables 2, 3, 4, and 5).
Roasting and Magnetic Separation
General
Magnetic separation is a simple, yet often effective, method of ore concentration usually considered in investigations concerned with the separation and recovery of iron minerals. Iron-hearing minerals that can be beneficiated by this means are (1) naturally occurring magnetite and (2) the paramagnetic products of the roasting of iron-bearing minerals.
Iron carbonate changes to a magnetic oxide by thermal decomposition. Carbon dioxide begins to evolve endothermically at about 300° C. to form FeO; an exothermic reaction follows, if oxygen is available for reaction with ferrous oxide. When iron carbonate is roasted in an inert atmosphere, some nonmagnetic ferrous oxide results; when it is roasted with full access to air, the nonmagnetic ferric oxide is formed. Best results are obtained under the weakly oxidizing conditions that exist when the furnace vents are open to the air but with no forced draft.
The ores investigated included limonite and siderite. Roasting research covered the individual ore types as well as composites of the two. Although the individual samples were considered as either limonite or siderite, they were in reality contaminated, one with the other, with the siderite containing a large amount of ferric iron and the limonite a lesser amount of ferrous iron (table 1, p. 3).
As preliminary work revealed that both the limonite and siderite could be roasted effectively at 1-inch size, all roasting tests were made on ore sized to minus 1-inch, unless otherwise specified. Except for a brief investigation of flash roasting, all roasts were made in an externally fired drum roaster, with provision for close control of both roaster temperature and atmosphere. This roaster is shown in figure 1.
The optimum conditions of roasting the limonite ores, as determined by controlled time, temperature, and furnace atmosphere variables, were found to be a 30-minute roast at 600° C. in carbon monoxide. These conditions were established by varying the temperature of the roast from 400° to 700° C. at 50° intervals; time of roast from 15 to 40 minutes; and atmospheres from carbon monoxide to hydrogen, propane, and mixtures of propane and air.
The optimum roasting conditions for the siderite ore were a 15-minute roast at 500° C. in the gases resulting from the decomposition of the ore. Temperatures of roast studied varied from 400° to 600° C. and roasting time from 5 to 30 minutes; furnace atmospheres used were atmospheric air, helium, and gases resulting from the decomposition of the ore.
Mixed limonite-siderite ores were subjected to an oxidizing roast before the reduction. Controlled variables of reduction were the same as for the limonite ore. Optimum conditions determined were a 20-minute roast at 550° C. in carbon monoxide.
Magnetic separations were made with a Wetherill high-intensity crossbelt separator, used as a rougher, and with a Davis-tube magnetic separator, used as a cleaner or finisher for the Wetherill magnetic concentrate. Other separators used were a Jeffrey wet drum, a high-intensity induced roll, and an a.-c., traveling-field, experimental unit made in the local laboratories.
Connors Brothers Deposit
Limonite
The limonite ore of this deposit was of low grade; the metallurgical sample contained 23.3 percent Fe and 50.2 percent acid insoluble matter. Most of the iron was present as limonite, with only 3 percent of the total iron as the carbonate.
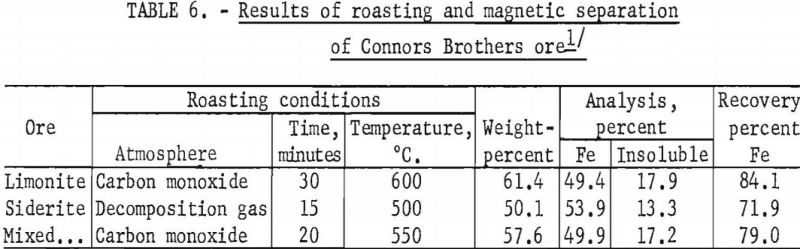
In a typical roasting test on this ore, magnetic ferroso-ferric oxide was formed by roasting for 30 minutes at 600° C. in an atmosphere of carbon monoxide. Magnetic separation in a Davis tube recovered 84.1 percent of the total iron in a concentrate containing 49.4 percent Fe and 17.9 percent acid insoluble material.
Siderite
Siderite ore from the Connors Brothers deposit was higher in iron content than the limonite ore. It contained 30.6 percent iron and 26.4 percent acid insoluble material. Of this total iron, however, only 71 percent was present as the carbonate.
When the ore was roasted for 15 minutes at 500° C. in the atmosphere resulting from the thermal decomposition of the carbonate (mostly CO2), only the siderite was converted to the magnetic ferroso-ferric oxide owing to insufficient reductant present in the furnace atmosphere to reduce a large portion of the limonite. Separation of the ore roasted under these conditions recovered 71.9 percent of the total iron in a concentrate containing 53.9 percent Fe and 13.3 percent acid insoluble material.
Mixed Limonite and Siderite
Since there was no sharp point of separation of the limonite and siderite in the deposit, the feasibility of roasting the mixed ores was investigated.
Roasting of a composite of equal weights of two ore types for 20 minutes at 550° C. in an atmosphere of carbon monoxide, followed by magnetic separation in a Davis tube, recovered 79.0 percent of the total iron in a concentrate containing 49.9 percent Fe and 17.2 percent acid insoluble matter.
Detailed results of these tests are given in table 6.
O. C. Pouns Deposit
Limonite
Limonite ore from the Pouns deposit with an iron content of 30.7 percent and 35.4 percent acid insoluble was the highest grade ore used in this investigation. Of the total iron in this ore, 8.0 percent was in the carbonate form.
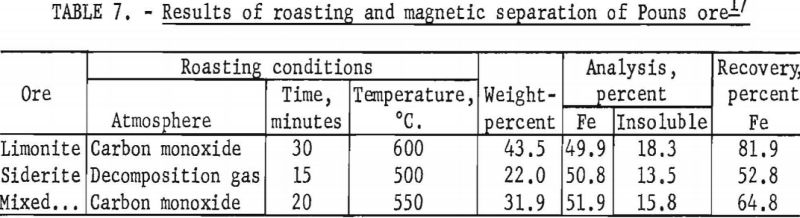
Roasting this ore for 30 minutes at 600° C. in an atmosphere of carbon monoxide, followed by magnetic separation at minus 100-mesh, resulted in the recovery of 81.9 percent of the total iron in a magnetic concentrate containing 49.9 percent Fe and 18.3 percent acid insoluble.
Siderite
In contrast to the limonite, the Pouns siderite was of very low tenor, containing 18.8 percent Fe and 45.8 percent acid insoluble. Of this iron, 63.3 percent was present in the ferrous form.
This ore was roasted under the previously determined optimum conditions of 500° C. for 15 minutes in an atmosphere composed of the gases produced by the decomposition of the ore. Magnetic separation recovered 52.8 percent of the total iron in a magnetic concentrate containing 50.8 percent Fe and 13.5 percent acid insoluble. The low recovery is due, in part, to the small percentage of total iron present in the ferrous state for the transformation to the paramagnetic form under roasting conditions employed and to the high content of iron-bearing clay in this ore.
Mixed Limonite and Siderite
Roasting of a composite of equal weights of limonite and siderite ores at 500° C. for 20 minutes in an atmosphere of carbon monoxide, followed by magnetic separation in a Davis tube, resulted in the recovery of 64.8 percent of the total iron in a magnetic concentrate containing 51.9 percent Fe and 15.8 percent acid insoluble. Table 7 gives the results of these tests.
Lee Ben Pynson Deposit
Limonite
The limonite ore from the Pynson property was also of very low grade, containing 21.3 percent Fe and 51.4 percent acid insoluble. It consisted almost entirely of ferric iron, with only 3 percent of the total being in the ferrous state.
The ferroso-ferric oxide formed by roasting at 600° C. for 30 minutes in an atmosphere of carbon monoxide was recovered by magnetic concentration in a Davis tube. This concentrate, containing 49.4 percent Fe and 17.5 percent acid insoluble, represented a recovery of 78.3 percent of the total iron in the crude ore.
Siderite
This carbonate ore was of even lower grade than the limonitic ore, with only 19.7 percent iron content and 45.3 percent acid insoluble. Of the total iron present, 64.5 percent was in the ferrous state.
Roasting for 15 minutes at 500° C. in an atmosphere consisting mainly of the gases resulting from thermal decomposition of the ore (carbon dioxide and water vapor), followed by magnetic separation, resulted in the recovery of 58.9 percent of the total iron in a concentrate containing 49.9 percent Fe and 12.7 percent acid insoluble.
Mixed Limonite and Siderite
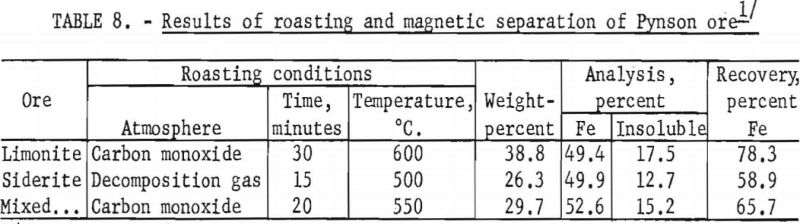
A sample containing equal weights of the two ore types was roasted for 20 minutes at 550° C. in an atmosphere of carbon monoxide and then magnetically separated. This treatment produced a magnetic concentrate containing 52.6 percent Fe and 15.2 percent acid insoluble, representing a recovery of 65.7 percent of the total iron. Results are tabulated in table 8.
Ettie Lattimer Deposit
Limonite
The limonite ore was the lowest in grade of all the limonitic ores investigated, with an iron content of 21.0 percent and an acid insoluble content of 53.5 percent. Like the other limonitic ores, it contained little ferrous iron,with only 3 percent of the total being in this reduced state.
Reduction roasting at 600° C. for 30 minutes in an atmosphere of carbon monoxide, followed by magnetic separation in the Davis tube, recovered 70.5 percent of the total iron in a magnetic concentrate containing 48.4 percent Fe and 19.4 percent acid insoluble.
Siderite
The sideritic ore from the Lattimer property contained 22.9 percent Fe and 39.7 percent acid insoluble. Of the total iron, 67.3 percent was in the ferrous state.
When roasted for 15 minutes at 500° C. in an atmosphere of decomposition gases and separated in a Davis tube, this ore responded, as other siderites in previous tests, with a low recovery of iron. The magnetic concentrates contained 49.2 percent Fe and 15.4 percent acid insoluble and represented 60.2 percent of the total iron.
Mixed Limonite and Siderite
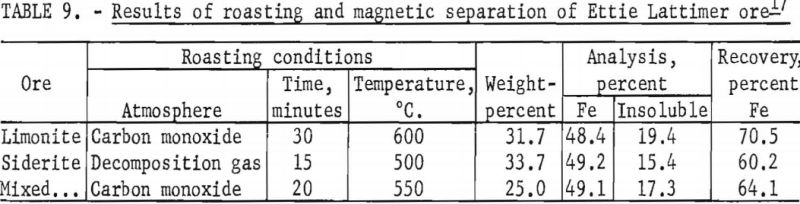
A composite of equal weights of the two Lattimer ores, when roasted at 550° C. for 20 minutes in an atmosphere of carbon monoxide, followed by magnetic separation, resulted in the recovery of 64.1 percent of the total iron in a magnetic concentrate containing 49.1 percent Fe and 17.3 percent acid insoluble. Summation of results is given in table 9.
Oxidation-Reduction of Mixed Ores
The investigation of roasting and magnetic separation, as applied to the several individual ores and to composites of both ore types from each property, considered only the variables of roasting time, roasting temperature, and furnace atmosphere. Further research to study other techniques, such as oxidizing the ore before reduction, wet versus dry magnetic separations, and the relative magnetic susceptibilities of magnetite and gamma-hematite, was conducted on a composite of equal weights of all eight of the ores.
The first approach to the problem of mixed-ore types, with their varied requirements for magnetic roasting, was by roasting in an atmosphere of ore-decomposition products. According to Mellor, it is probable that ferrous carbonate first splits into ferrous oxide and carbon dioxide and that these react on one another to give ferric oxide and carbon monoxide. The intent of this roasting was to use the carbon monoxide released from the thermal decomposition of siderite to reduce the limonitic iron oxides. This technique was not successful, as the amount of magnetic iron oxide produced was barely sufficient to account for the iron originally contained in the siderite. Analysis of the exit gases disclosed no carbon monoxide. The presence of 0.2 to 0.4 percent of free carbon in the roasted ore suggests that carbon monoxide was combining to produce carbon dioxide and free carbon instead of reducing the iron oxides in the ore.
The second approach to this problem was through an oxidizing roast before the reduction roast. When roasted in air, both ferrous carbonate (siderite) and the hydrated iron oxides (limonite) are converted to ferric oxide, which is readily reduced to the magnetic iron oxide, magnetite.
Reduction roasting and magnetic separation of this uniformly oxidized material showed improvement in both grade of concentrate and recovery over that obtained when the different ore types were treated without a prior oxidizing roast.
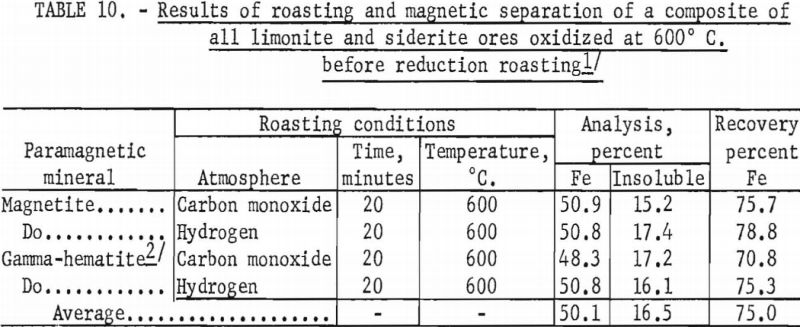
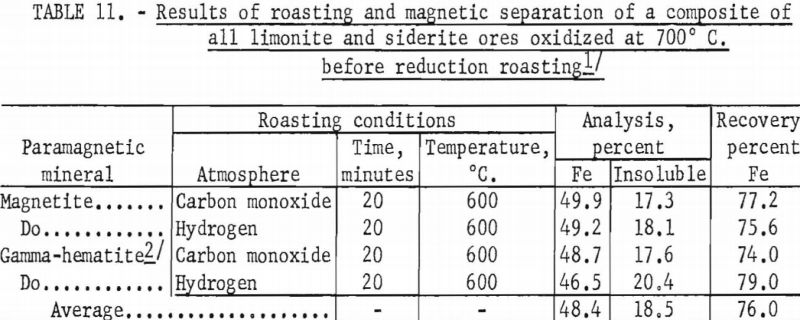
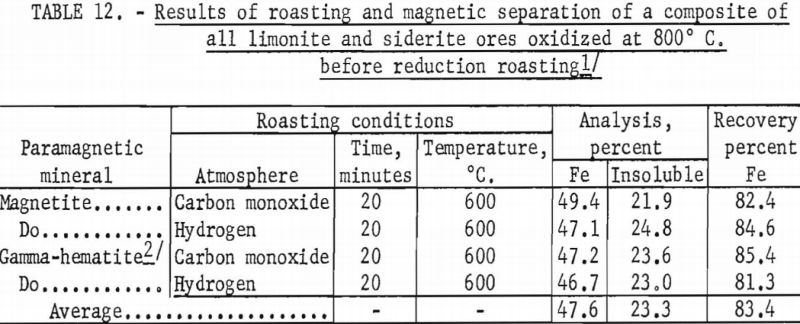
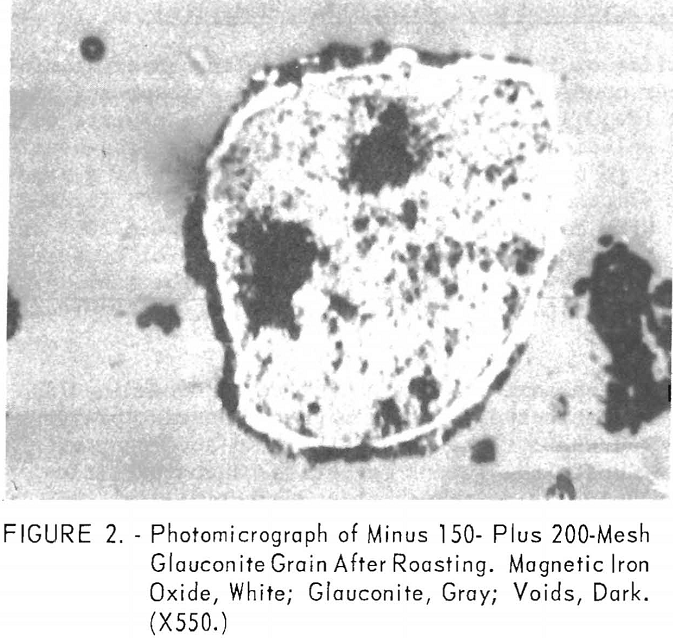
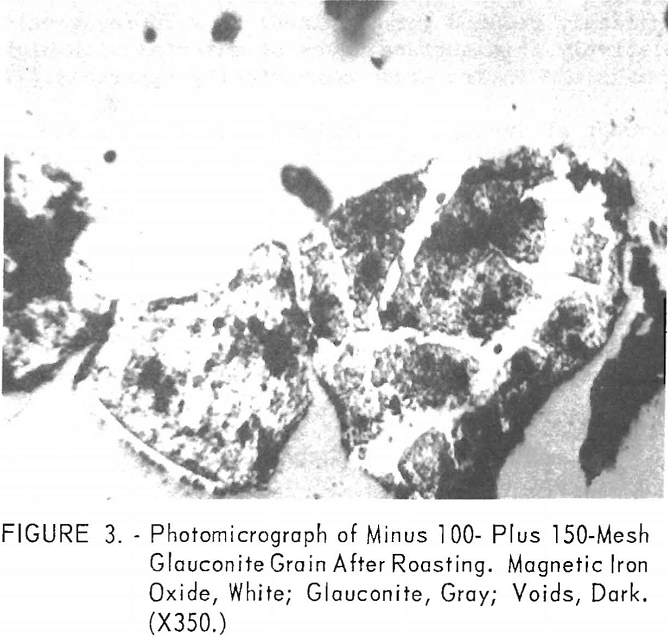
In a typical test, the ore was first roasted in air at 600° C., then reduced at the same temperature by passing a stream of carbon monoxide through the roaster. The reduced ore was cooled in an inert atmosphere to prevent reoxidation. The temperature of the oxidizing step was shown to have a decided influence on both grade and recovery of the concentrate derived from the reduced ore (tables 10, 11, and 12). Processing at 600° C. resulted in the highest grade concentrate and lowest recovery (50.9 percent Fe and 15.2 percent acid insoluble with a recovery of 75.7 percent), while the ore roasted at 800° C. showed the lowest grade and highest recovery (49.4 percent Fe and 21.9 percent acid insoluble with a recovery of 82.4 percent). The higher recovery results from the recovery of altered glauconite (hydrous silicate of iron and potassium) grains that have become susceptible, in part, to reduction to magnetic iron oxide after a preoxidation treatment at 800° C. The galuconite was, in effect, magnetic but of low iron content. The micrographs of roasted glauconite grains (figs. 2 and 3) show the magnetic iron oxide as white coatings and stringers in a grey matrix of glauconite, thus emphasizing the impracticality of grinding to the degree necessary to liberate this iron oxide.
Comparison of Magnetite and Maghemite (Gamma-Hematite)
A series of magnetic oxides of iron may be formed from iron-bearing minerals by roasting under proper conditions of temperature and atmosphere. Magnetite (Fe3O4) and maghemite (Fe2O3) are the end members of what appears to be a continuous series of solid solutions. 14/ Reduction roasting of iron-bearing minerals in hydrogen from 400° to 570° C. results in the formation of a product with, or similar to, the composition of magnetite; above 570° C. ferrous oxide is likely to form. When magnetite is heated in air between 200° and 400° C., it takes in oxygen, the rate and extent being functions of temperature, and the composition approaches that of gamma-hematite (maghemite). This change is accompanied by a substantial increase in volume (7.76 percent), if the final product is maghemite.
In this research, magnetite was oxidized to maghemite to determine which form of the oxide would exhibit the greatest magnetic susceptibility when very finely divided and (2) whether the large expansion accompanying gamma oxidation would tend to loosen the locked magnetite-gangue particles. Results of the study were inconclusive as to magnetic susceptibility. Increased liberation of the gangue particles by the oxidation expansion was not significant, but the oxidation did improve the grindability of the roasted ore.
Flash Roasting
Effective magnetic separation of iron-bearing minerals does not require that the mineral grain be completely reduced (or oxidized) to a ferromagnetic form. The formation of a relatively thin surface layer of material with high magnetic susceptibility will make the entire grain magnetically separable.
A brief study of this concept of incomplete magnetic roasting was made to determine if the process was technically feasible for the east Texas iron ores. Roasting was accomplished in a vertically mounted electric tube furnace equipped with baffles to control the retention time of the ore and a water seal at the base to permit control of the roasting atmosphere.
At 700° C. in an atmosphere of carbon monoxide, minus 65-mesh ore was reduced in approximately 2 seconds. The reduction appeared to be complete throughout the grains. On minus 6- plus 65-mesh ore, under the same roasting conditions, 7 seconds were required to reduce a surface film. This reduction
made the particles magnetically separable, but further grinding destroyed the film, leaving a core that was not recoverable by magnetic means. Concentrates obtained from this flash-roasted material were of acceptable grade (46 percent Fe and 20 percent acid insoluble), but recovery was limited to 51 percent. One of the chief deterrents to flash roasting is the difficulty of grinding the crude ore to the necessary degree of fineness. On ore fractions, such as washer tailings, that would not require grinding, this roasting may prove valuable. As applied to washer tailings obtained in this research, a magnetic concentrate containing 39.9 percent Fe and 27.6 percent acid insoluble was recoverable. Recovery at this grade was only 16 percent of the total iron, but the feed contained only 15.8 percent Fe, most of which was present as slimes.
