Table of Contents
The main source of beryllium in pegmatite is the mineral, beryl. Many of the conventional methods for the determination of beryllium in beryl are not suitable for control purposes because of lack of speed and accuracy, especially in the range below a few tenths percent. Beryl resists the usual chemical attack and can be lost in a hurried chemical analysis.
The spectrograph offers aid in this range of composition; however, to be accurate, rapid spectrographic methods require a uniform matrix. In the present study, the material to be beneficiated is composed of 4 major minerals and, depending on the geological history and previous separations, some 4 to 7 minerals in small quantities (see table 1). Any of the four major constituents are expected to appear singly or in combination in the samples submitted for analysis.
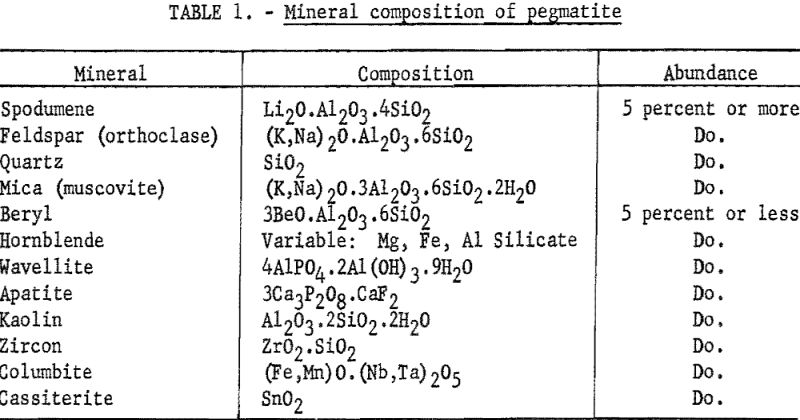
The heavy minerals, cassiterite, columbite, and zircon, if present in any considerable quantity, are removed by tabling before further beneficiation is attempted. The other components that are present can introduce a matrix error to a spectrographs determination of beryllium.
Experimental
To determine the amount and direction of matrix error, a series of synthetic standards at the 0.002- and 0.10-percent Be levels was compounded, using a carefully analyzed beryl as the source of beryllium and pure, beryllium-free spodumene, mica, quartz, sodium feldspar, potassium feldspar, and kaolin as the various matrices. This series was analyzed for beryllium by using a set of synthetic standards covering the range from 0.002 to 0.42 percent Be in a base composed of equal parts of spodumene, mica, feldspar, and quartz.
Several methods were tried. A solution method was quickly abandoned because of the difficulty of solubilizing the beryl completely. Results of the study are presented in table 2. All results are the average of either 8 or 16 determinations.
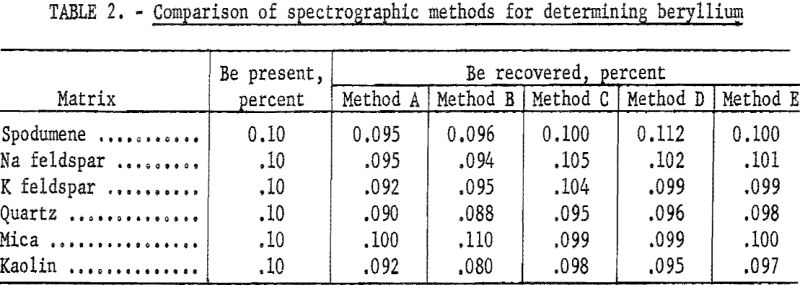
Method A: Equal portions of sample and pure, flake graphite were burned to completion in a d.c. arc at 250 volts, 12.5 amperes.
Method B: Equal portions of sample and flake graphite were pelletized and excited in the a.c. spark.
Method C: This method was essentially the same as described by Jaycox.
Powdered samples were mixed thoroughly with varying quantities of copper oxide and graphite and burned to completion in a d.c. arc at 250 volts and 10.0 amperes. The data in table 2 are for a sample-to-buffer ratio of 1:10. At this dilution the method gave excellent results and was studied at some length. Reproducibility and accuracy were found to be satisfactory. Deviation was about 5 percent of the quantity present for the average of duplicate determinations. However, the sensitivity was not adequate. The lower limit was about 0.010 percent Be, and in the lower range reproducibility was not good, probably because of the difficulty of proper sampling and mixing.
Method D: Zander and Terry and Churchill and Russell among others have described methods that use a dry flux, sample and flake graphite as a pellet with spark excitation. This general method was tried with sample-to-flux ratios ranging from 1:4 to 1:10, using lithium fluoride, sodium fluoride, or sodium tetraborate as the flux. At the 1:10 ratio (reported in table 2) sensitivity was not adequate. At any lower ratio of sample-to-flux, matrix effects were not suppressed enough. For the higher ranges of analysis the method appeared to be excellent.
Method E: Hartleif and Kornfeld (translations of these two papers are available from Henry Brutcher, 3185 N. Marengo, Altadena, Calif.) Priced and Hasler and Barley describe a fusion-spark method in which the powdered sample is added to a mixture of a flux and an internal standard, fused, ground, pelletized with graphite and excited in an a.c. spark. The data presented in table 2 (method E) are averages of either 8 or 16 determinations by this general method. Investigation showed that, with most of the available fluxes, sample-to-flux ratio could be as low as 1:4 without allowing serious matrix errors. Sensitivity appeared to be adequate.
Because of the many steps involved in sample preparation, this method appeared too time-consuming for control purposes. However, since the method seemed to provide the best solution to the problem, mechanical adaptations were attempted wherever possible to reduce the time necessary for sample preparation to less than 1 hour. The technique described in the next section was adequate.
Sample Preparation
Pellets made with carbonates or carbonates mixed with fluorides, borates, or hydroxides were physically unstable in the medium-to-heavy spark found to be necessary in the low range of analysis. Sodium tetraborate, lithium fluoride, lithium hydroxide, sodium fluoride, sodium hydroxide, potassium hydroxide, and potassium pyrosulfate were studied as possible alternate fluxing agents.
Lithium compounds were not used because an occasional semiquantitative estimation of lithium in the samples was anticipated. Lithium fluoride, lithium hydroxide and mixtures of either of these with lithium tetraborate, sodium tetraborate, or boric acid proved to be excellent fluxes, however, and can be used under different circumstances. Hydroxides, in general, were found to be somewhat difficult to recover quantitatively from a crucible. Potassium pyrosulfate gave poor reproducibility in the spark. Anhydrous sodium tetraborate was found to be a satisfactory flux.
Anhydrous sodium tetraborate rehydrates slowly on exposure to moist air. To determine the effect of moisture on the reproducibility of the method, sample-flux mixtures were fused, ground, and exposed to an atmosphere saturated with water vapor at room temperature for 15, 30, 60, and 120 minutes. Pellets made after 60 minutes exposure to these conditions were physically unstable in the spark, and reproducibility was poor. Weight increased only 0.30 percent. However, exposures to saturated water vapor for less than 60 minutes apparently had no effect on accuracy or reproducibility. Weather in the Southeast made it advisable to handle each replicate in a stoppered vial. Finished pellets absorbed only a trace of moisture and needed no protection.
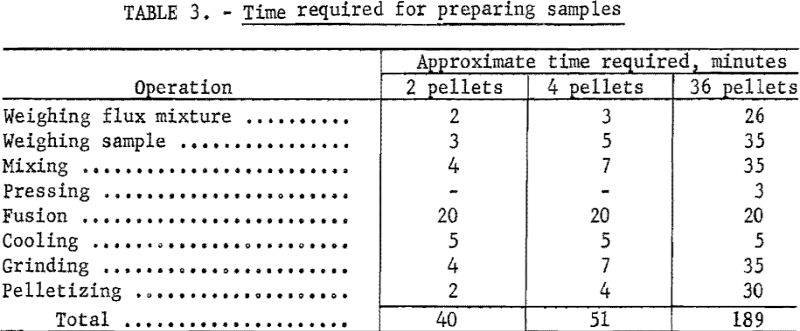
Each step of sample preparation was studied separately with regard to minimum time requirements without loss of accuracy of the method.
A mixture of sodium tetraborate, internal standard, and flake graphite was a satisfactory fluxing material. Sodium tetraborate is a quiet flux, and no spattering occurred at the fusion temperature. Because of poor contact of sample and flux in the presence of graphite, fusion was not complete, but microscopic examinations of fusion products showed that more than 90 percent of the crystal structure of the sample was destroyed after 20 minutes of heating. This was enough to remove nearly all matrix effects due to crystal structure. A difference in particle-size distribution between sample and standard introduced a small error, apparently due to differences in the reaction of sample and flux. When sample preparation is reasonably reproducible, this error is negligible. In unfavorable cases, the error could be removed by compacting the sample-flux-graphite mixture by hand before fusion. Errors caused by extreme particle-size differences were removed by fusing only sample and flux mixture. Graphite could then be added during the subsequent grinding operation.
Two methods were used for grinding the fusion product. In the preferred method, the sample was removed from the furnace, allowed to cool for 5 minutes, and then transferred to a tool-steel vial that was adapted for use with a dental amalgamator. Two steel balls were used as the grinding medium. Grinding required 20 to 30 seconds. To prevent cross contamination, the vial was wiped clean with a pipe cleaner between samples. Alternatively, the product was ground in a few seconds by hand in an alundum mortar. However, the mortar required washing and drying between samples, and the time requirements were excessive.
Pellets were made on a pelleting press. It was necessary to hold the compression cycle for not less than 10 seconds at a point near the maximum pressure limit of the dies at the graphite-to-powder ratio of about 1:1 used throughout this study.
The time requirements for each step of sample preparation are listed in table 3. The times listed are approximate averages of the requirements of four technicians in this laboratory.
The Method
The equipment used in this study is listed in table 4.
Two flux mixtures and two sets of sparking parameters were necessary to cover the expected range of analysis.
To cover the low range (0.002 to 0.20 percent beryllium) the flux mixture was compounded as follows: Screen a dry, analytical-grade, ground borax glass on a 65-mesh screen, and discard the oversize. Grind a weighed portion of powdered vanadium pentoxide with a small portion of the undersize borax until it is completely dispersed, and then dilute with the borax to 0.5 percent by weight V2O5, Dry at 530° C. for 1 hour, crush, and brush through the 65-mesh screen. Dilute with pure, minus-100-mesh flake graphite in the ratio of 1:1 and mix. Label “Flux-Low Range” and store in an oven held above 80° C. until used.
Weigh into glass shell vials as many 0.360-gram portions of the flux mixture as will be needed. Weigh in duplicate 0.040-gram portions of minus-200-mesh sample into the vials, mix on a dental amalgamator, transfer to individual ½- by ½- inch graphite crucibles, press by hand with a stainless steel rod, and fuse at 760° C. 20 minutes. Cool, transfer to a tool-steel grinding vial adapted for use with a dental amalgamator, grind 30 seconds (dry 45 minutes at 90° C. at this point if the fusion product has been exposed to the atmosphere for more than an hour), transfer to the die of a pellet press, and make pellets at 150,000 pounds per square inch with the compression cycle held for 10 seconds.
To cover the high range (0.100 to 5.0 percent beryllium), the flux mixture is compounded as follows: Screen a dry, analytical-grade, ground borax glass on a 65-mesh screen and discard the oversize. Grind a weighed portion of powdered vanadium pentoxide with a small portion of the undersize borax until it is completely dispersed and then dilute with borax to 1,5 percent by weight V2O5. Dry at 530° C. for 1 hour, crush, brush through the 65-mesh screen, dilute 1:1 with pure, minus-100-mesh flake graphite, and mix. Label “Flux – High Range” and store in an oven held above 80° C, until used.
Weigh into glass vials as many 0.400-gram portions of the flux mixture as will be needed. Weigh 0.005-gram portions of sample in duplicate into the vials, mix on a dental amalgamator, transfer to individual ½- by ½-inch carbon crucibles, press by hand with a stainless steel rod, and fuse at 760° C. for 20 minutes. Cool, transfer to a tool-steel grinding vial adapted for use with a dental amalgamator, grind for 30 seconds (dry 45 minutes at 90° C. at this point if the fusion product has been exposed to the atmosphere for more than an hour), transfer to the die of a pellet press, and make pellets at 150,000 pounds per square inch with the compression cycle held for 10 seconds.
The pellet is the lower electrode. The upper electrode is 0.180-inch, regular grade, medium porosity graphite with the end ground to 120° included angle.
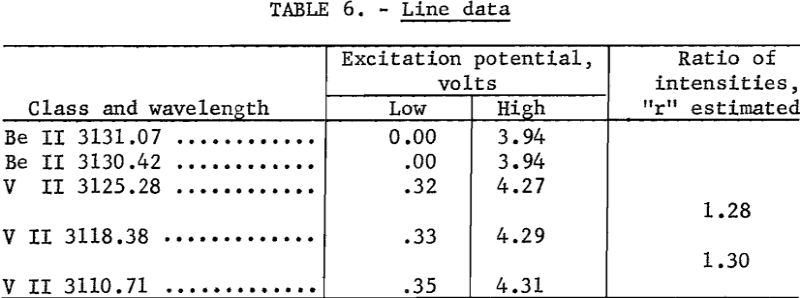
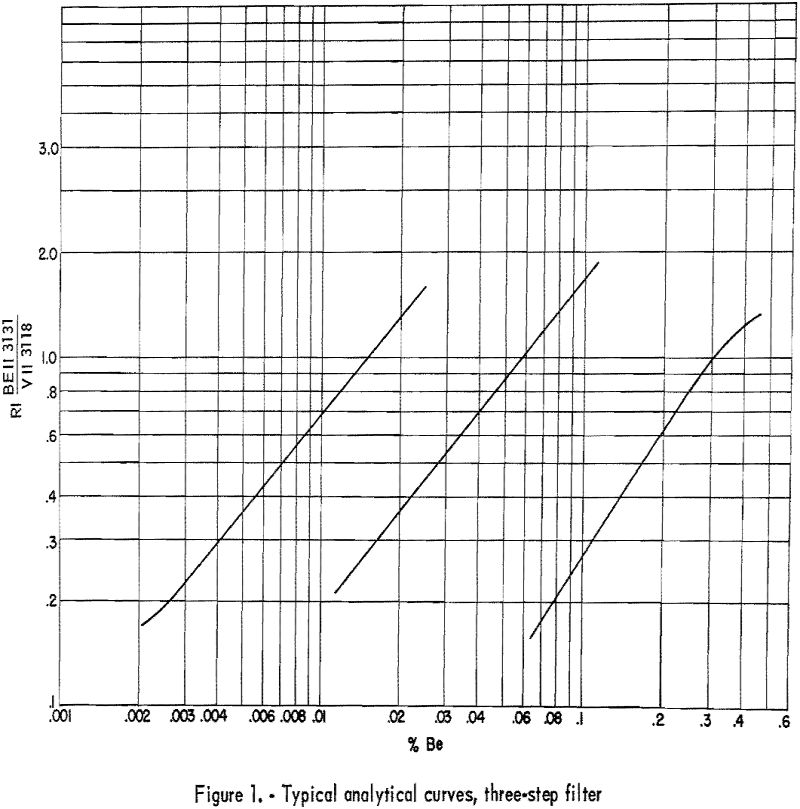
Develop plates 3½ minutes in Eastman X-ray developer, and fix 2 minutes in Eastman F-5. Wash 2 to 4 minutes, depending on the temperature of the wash water. Record microphotometer readings (percent transmittance) for Be II 3131.07 and any 2 of the following; V II 3110.71, V II 3118.38, or V II 3125.28. Any one of the three V II lines is suitable as an internal standard line, and any adjacent pair is suitable for plate calibration by the two-line method as described by Church-hill. Background, if present, is subtracted on the microphotometer by balancing adjacent to the line rather than on an unexposed portion of plate. Because of the many minor fluctuations that may occur in preparing samples, it is recommended that the slope of the analytical curves be corrected daily and that the shift of the curves be corrected for each plate. These corrections can be made by including on each plate a number of synthetic-beryllium standards made by successive dilutions of a carefully analyzed beryl with a matrix that approximates that of the unknown samples. Typical analytical curves are shown in figure 1. Line data are given in table 6. Wavelength and excitation potential are taken from Moore. Values of “r” were estimated in this laboratory.
Accuracy and Reproducibility
Four samples containing 0.094, 0.50, 1.98, and. 4.00 percent beryllium, respectively, were available as checks on accuracy. These analyses of samples by four independent laboratories are considered to be correct.
Working standards were prepared by diluting a carefully analyzed beryl with a matrix composed of equal portions of spodumene, feldspar, quartz, and mica. Assuming the working standards to be correct, duplicate determinations were made of the beryllium content of the standard samples. Data are presented in table 7.
Probable error of duplicate determinations were calculated according to the formula:
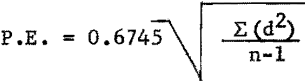
where d = deviation from mean,
n = number of replicate determinations,
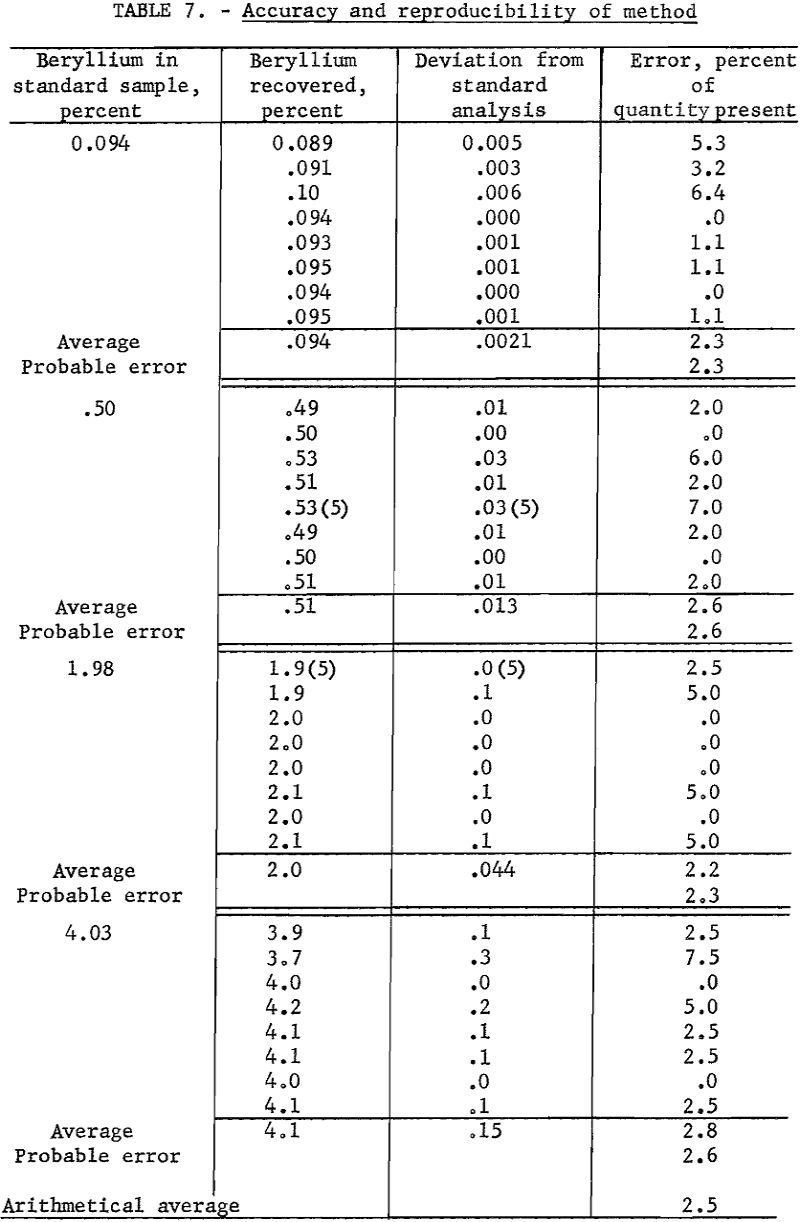
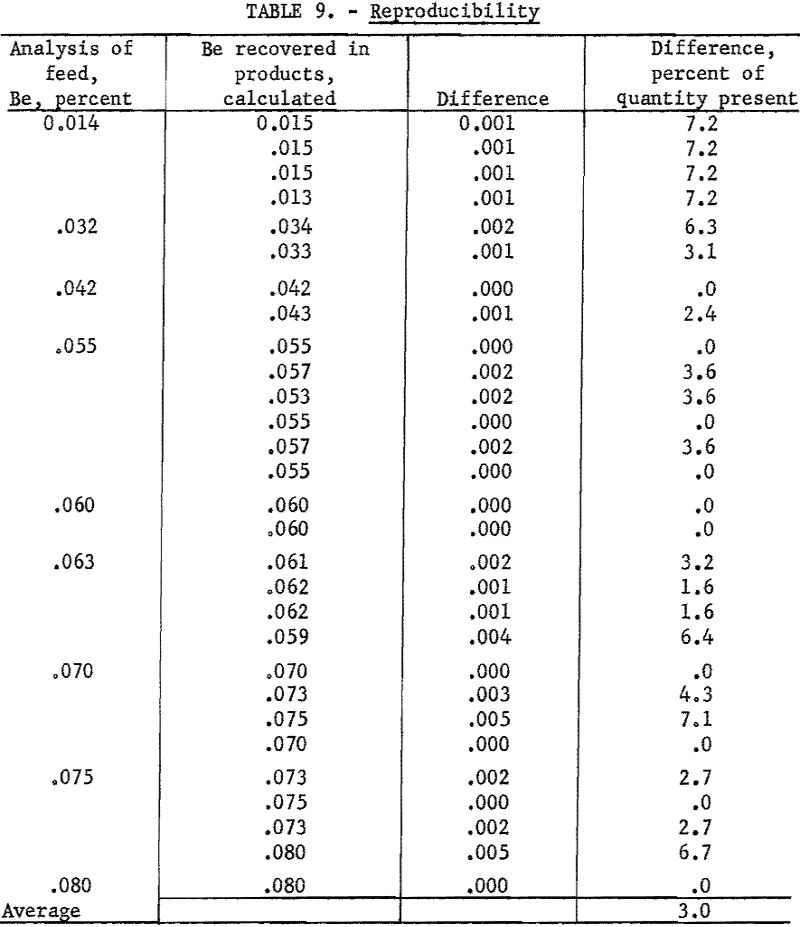
As an overall check on reproducibility, a group of samples representing individual separation tests was calculated back to feed analysis. This method of calculation is illustrated in table 8. Nearly all the products were analyzed at 1 time on 1 plate. The analysis of the feed was made spectrographically by this method but not necessarily at the same time as those of the products.
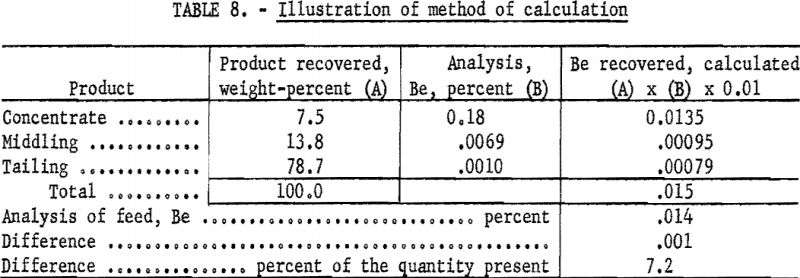
Results of 29 of these calculations are listed in table 9. Note that, in this series, deviations include errors of recovery, sampling, and weighing that originate outside the spectrographic laboratory.