Table of Contents
As part of the U.S. Department of the Interior, Bureau of Mines, mission to assure a viable domestic minerals and materials economy, research is being conducted to investigate methods for recovering accessory elements from solutions resulting from hydrometallurgical processing of base-metal sulfide concentrates. These types of sulfide concentrates have traditionally been treated by smelting procedures because they efficiently recover the accessory elements (Ag, Au, Bi, Cd, In, Se, Te, As, Sb, and Tl) as well as the major elements (Pb, Zn, Cu, and Ni) contained in the concentrates. However, recent concern over air quality deterioration due to smelter emissions has stimulated investigation of potentially less air polluting hydrometallurgical alternatives to smelting. Certain procedures investigated appear to be competitive with smelting for the recovery of the major elements contained in base-metal concentrates but none have advanced sufficiently to enable complete and efficient recovery of the accessory elements that occur in sulfide concentrates. Recovery of these elements is desirable from an environmental standpoint and because many of them constitute the only domestic source of these commodities or are important to the overall economics of the process sequence.
Chloride-based hydrometallurgical procedures have received considerable attention for treatment of base-metal sulfide concentrates because they are adaptable to a wide variety of concentrates. Usually ferric or cupric ions, or oxygen or chlorine are employed to oxidize the sulfur content of the metal sulfides to form elemental sulfur, thus freeing the metallic constituents formerly present in the sulfide minerals and allowing them to be solubilized by the chloride media. Silver can be a valuable constituent of these leach solutions, and its rejection or selective recovery is highly desirable because it inevitably follows copper through any subsequent copper reduction-recovery process.
Because chloride-based hydrometallurgical processes for treatment of sulfide concentrates are recent innovations, little is known regarding selective recovery of silver from complex leach solutions. Liddell reported an iodide precipitation procedure for silver recovery from mixed sulfate-chloride roast-leach solutions; however, the solutions were too dilute to represent many of the more recent hydrometallurgical leach solutions.
This report describes results obtained in investigating the effectiveness of iodide precipitation for recovery of macro amounts of silver in chloride-based hydrometallurgical leach solutions.
Procedure & Equipment
Silver iodide precipitation experiments were conducted on a pregnant solution obtained by ferrous chloride-oxygen leaching of a copper-silver-bearing sulfide concentrate. The composition of the major elements contained in this chloride solution, which had a pH of 1.5, is shown in table 1.
The method employed in the precipitation and iodide regeneration experiments was to pour the proper amount of iodide solution into a rapidly stirred pregnant solution. After 5 minutes, the stirring rate was reduced to allow the fine Agl particles to agglomerate into flocs. Stirring was such that motion was maintained throughout the solution, but the larger flocs were unlevitated. Flocculation required 30 minutes. The flocs were allowed to settle for 60 minutes before decanting and filtering the supernatant solution to recover the small amount of Agl fines that failed to settle. The settled Agl was also filtered to dewater it. Both filter cakes were washed with water and dried. They were combined, ground to-powder, and suspended in water by stirring. Solid Na2S was then slowly added to the slurry until the pH rose to 10.5. This produced Ag2S and regenerated the NaI.
Metal concentrations were determined by a variety of methods. Solutions were analyzed by atomic absorption spectrophotometry and a radiotracer technique utilizing Ag tracer. Solid Agl and Ag2S products were analyzed by optical emission spectroscopy. More accurate determinations for copper and iron in Ag2S were made by atomic absorption spectrophotometry. The iodide content of the Ag2S was monitored by the following procedure: a 0.10-gram portion of the Ag2S was added to 10 ml of 5 M HNO3 containing 0.2 to 0.3 gram KMnO4 and heated to 90 C for 0.5 to 1 hour, and treated with H2O2. This treatment oxidized the Ag2S to Ag2SO4 and the AgI to AgIO3. Subsequent H2O, treatment resulted in reduction of the remaining MnO4 to Mn2+ and reduced the AgIO3 to AgI. The amount of iodide was calculated from the weight of the AgI precipitate.
Results and Discussion
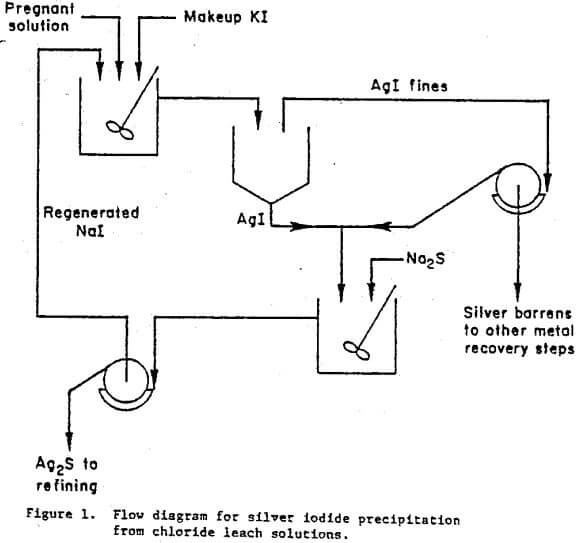
A conceptual flow diagram illustrating the essential details of the iodide precipitation and regeneration technique is shown in figure 1. Silver chloro-complex reacts with sodium iodide to form insoluble AgI and dissolved NaCl,
2(AgCl4)3- + 2NaI → 2AgI + 2NaCl + 6Cl-…………………………….(1)
Silver iodide is recovered after agglomeration by a combined decantation-filtration procedure. The AgI is then converted to Ag2S by reaction with relatively inexpensive Na2S,
2AgI + Na2S → Ag2S + 2NaI………………………………….(2)
This regenerates the more expensive iodide precipitant for recycling to fresh silver-bearing solution. The Ag2S precipitate can then be treated by cementation on 4-mesh aluminum chips in a solution of NaOH as was practiced commercially at the Nipissing Mining Company of Ontario, Canada.
Chemical reactions generally ascribed to this procedure are as follows:
2Al + 2NaOH + 2H2O → Na2Al2O4 + 6H,………………………………………(3)
6H + 3Ag2S + 6NaOH → 3Na2S + 6H2O + 6Ag……………………………….(4)
The crude silver product is then fire refined to a marketable grade of silver.
High efficiency of iodide precipitation and high return of NaI indicate that the precipitation and regeneration reactions proceed principally according co equations 1 and 2. Losses of iodide are due mainly to formation of small amounts of Cu(I) iodide complexes and I2 or ICl2 .
Initial experiments were conducted on a batch basis to determine the amount of iodide required to precipitate the silver content of the feed solution. These experiments showed that it was necessary to maintain the pH of the solution below 2.0 in order- to preclude contamination of the AgI with coprecipitated Fe(OH)3. Results of these experiments are presented in figure 2. Precipitation was nearly quantitative until over 90 pct of the silver had been precipitated. Additional iodide was required to achieve 100-pct precipitation of the silver. This occurred at a 20-pct excess of iodide over the stoichiometric requirement. Neither temperature nor the cation employed in the iodide salt had an observable effect on the silver recovery. However, temperature strongly influenced the agglomeration characteristics of the precipitate particles. Flocculation did not occur at 49° C, was poor at 34° C, but occurred readily at 21° C. Thus, temperature must be controlled to promote good flocculacion.
The iodide regeneration concept was tested experimentally at room temperature on AgI recovered from the precipitation experiments and found to be feasible. These experiments indicated that regeneration was promising because 95 pct of the iodide was solubilized in 10 minutes at pH 10.5 with only a 5-pct excess of Na2S.
An experiment employing 8-liter batches of the pregnant feed solution was conducted to determine the overall silver recoveries and iodide losses to be expected when the iodide precipitant is recycled. The experimental procedure was modified in three ways to allow for the necessary sampling. First, small amounts of AgI fines that did not flocculate and settle were washed and then leached with 200 ml of 0.03 M NaCN solution. This was done to determine the amount of silver contained in the fines. Next, the bulk AgI precipitate was washed with water, dried at 120 °C, and then weighed before converting it to Ag2S. Then the 50 to 80 ml of the filtrate and washings from the Ag2S precipitation, which contained the recovered NaI, was treated with sufficient makeup KI (KI is currently less expensive per mole of contained I than NaI) and then reused to precipitace the Agl from a second 8-liter batch of pregnant solution. The second Agl precipitacion, isolation, and conversion to Ag2S, was conducted in the same manner as the first. The recovered NaI and makeup KI were likewise used to treat a third 8-liter batch of pregnant solution. By continuing in this manner, a total of five successive AgI precipitation-conversion to Ag2S-NaI recovery cycles were conducted.
The average mass balance results of the 5-cycle precipitation-regeneration experiment are shown in figure 3. An analysis of the Ag2S product is shown in cable 2. These results confirm results obtained in batch tests that indicated 92 pct silver precipitation at 100 pct stoichiometric iodide addition. Iodide losses in the barren solution, in the Ag2S, and in the AgI fines amounted to 8.8 pct. However, the iodide lost to the fines would not be lost in practice because the Agl fines would be recovered by filtration and mixed with the agglomerated AgI for treatment with Na2S. This would reduce the iodide losses to 4.8 pct from the 8.8 pct shown in the cable. There is some possibility that the dissolved silver and iodide lost in the barren solution would return in the next batch of pregnant solution. However, this would be a characteristic of the specific application and plant flowsheet. It is anticipated that in most cases the barren solution would be treated for other metal recovery, and some of the solution would be bled to control impurity buildup and used to leach fresh ore or concentrate before returning for another iodide treatment for silver recovery. Because of the uncertainty of silver and iodide recycle in the barren solutions the silver and iodide remaining in this solution was considered to be completely lost, as shown in figure 3. The major impurities in the Ag2S product represent less than 2.5 pct of its total weight. This low-impurity content favors a simple procedure for processing to metallic silver.
The combined filtrate and washings from the Ag2S precipitation contain the recovered NaI. Any sulfide remaining in this solution will, on addition to another batch of pregnant solution, be converted to elemental sulfur by Cu(II) or Fe(III), and thus precipitate with the AgI. If necessary, the excess Na2S can be removed by treating the NaI solution with ferrous chloride to precipitate FeS according to
FeCl2 + Na2S → FeS + 2NaCl……………………………(5)
Reduction of the pH to 7.0 or below is evidence that this reaction has gone to completion. If the presence of additional iron in the Ag2S is not objectionable, FeCl2 can be added to remove excess sulfide before filtering the silver sulfide.
The procedure was further tested by adding stoichiometric amounts of iodide directly to other silver-bearing chloride leach solutions. Over 90 pct of the silver was recovered in each case. Solutions tested included a solution containing, in grams per liter, Cu(0.2), Fe (31), Zn (2), Pb (5), Ag (0.05), and Cl (160), which was derived from leaching a galena concentrate with acidified ferric chloride. A solution containing, in grams per liter, Cu (0.4), Fe (0.02), Zn (200), Pb (0.4), Ag (0.05), and Cl (200), was derived from leaching a sphalerite concentrate with chlorine-oxygen. A solution containing, in grams per liter, Cu (19), Fe (60), Ag (0.07), Cl (97), was derived from ferrous chloride-oxygen leaching of a copper sulfide concentrate. These results indicate that the iodide precipitation technique has wide applicability for treating oxidized chloride leach solutions for silver removal.
Silver contained in oxidized chloride process leach solutions can be recovered by an iodide precipitation technique that involves addition of a soluble iodide salt to precipitate the silver content as AgI. The AgI precipitate is created with Na2S to form Ag2S and regenerate the iodide precipitant. Silver recovery as high as 100 pct can be achieved by this procedure although 90- to 92-pct recovery represents a more practical balance between silver recovery and iodide losses. Effective flocculation of the AgI is achieved by operation near 21 °C with gentle stirring of the AgI precipitate. A seeding time of 60 minutes allows 95 pct of the AgI to be recovered by decantation of the silver barren solution. Iodide losses are expected to be 4.8 pct of the amount fed to the pregnant solution per precipitation-regeneration cycle.