Table of Contents
- Production Methods For Chromium Metal
- Carbon Reduction of Chromium Oxide
- Chromium by Dissociation of Chromium Nitride
- Fusion Electrolysis
- Chromium from Amalgam
- Dissociation of Chromium Iodide
- Chromium Oxide Reduction with Metals
- Hydrogen Reduction of the Chlorides
- Chromium Chloride Reduction by Metals
- Reduction of Chromium Chloride with Magnesium
- Purification of High-grade Chromium by Hydrogen Treatment
- Production of Pure Chromium from Chloride on a Commercial Scale
Aluminothermic or silicothermic processes used for the metallurgical production of chromium metal from chromium oxide yield a metal that may contain more than 99 percent chromium. A metal of this purity apparently meets all the requirements of the alloy industry, but the price is about three times that of chromium in the form of low-carbon ferrochrome, which indicates that the economics of the reduction processes to produce a metal of this high purity are not entirely satisfactory, as compared to the methods for making the ferro-alloy. This high-grade product is still not pure enough to yield a malleable metal.
None of the described processes is used at present for producing high-purity chromium on a commercial scale. Reduction of the chloride with hydrogen, combined with recent hydrochloric reclaiming processes, is promising, but it has not advanced to the stage of commercial production.
The reduction of the chloride with magnesium, followed by vacuum separation of the salt, has been developed far enough so that it could be used on a pilot-plant scale whenever large quantities of pure trichloride can be produced.
The purification of slightly oxide-contaminated chromium by dry hydrogen at high temperature by the use of zirconium hydride as a gasometer and as a circulation pump yields a very soft and hot malleable chromium, which, however, is brittle when cold.
It was desired to produce a high-purity metal so that its malleability could be studied. One of the authors has already described the malleability of chromium as produced by calcium reduction of pure chromium oxide in a bomb or by reducing chromic chloride in the same manner. This metal was hot malleable to some extent, which confirms a statement by Marden, but it was brittle when cold. The reasons why chromium is brittle are not known, but, in the light of investigation by the authors on other so-called brittle metals, it may be assumed that the presence of soluble oxide, perhaps also nitride, my be the contributing cause of the lack of ductility.
An excellent survey concerning the malleability and physical properties of various kinds of electrolytic chromium was made by Brenner, et al, of the National Bureau of Standards, but no conclusion was arrived at as to the causes of the brittleness.
Production Methods For Chromium Metal
Carbon Reduction of Chromium Oxide
Raukloh has shown that chromium metal may be obtained by reducing chromium oxide with carbon in a vacuum. Schecht improved this method by operating in a partial vacuum with hydrogen and claimed that the rate of reduction was increased in a partial vacuum.
The possibility of reducing chromium oxide with carbon in vacuo was investigated by Kroll and Schlechten. A chromium metal with 97 percent chromium and 0.09 percent carbon could be obtained, but the metal loss by volatilization in the high vacuum was considerable . This method does not permit obtaining malleable chromium, as some unreduced oxide remains behind in the metal.
Chromium by Dissociation of Chromium Nitride
Many nitrides are easily prepared by- the reaction of anhydrous chlorides with ammonia gas at about 550° C. Ammonium chloride also is formed and must be reclaimed. A number of nitrides may be dissociated at elevated temperatures in vacuo.
Chromium nitride may be prepared by treating chromous or chromic chloride with ammonium chloride in nitrogen or in an inert atmosphere. When chromium nitride is heated to 1,400° C. in vacuo, nitrogen is evolved, and a metal containing more than 93 percent chromium may be obtained in this manner. Only a few tenths percent nitrogen remain after degassing at 1,400° C. in a vacuum of a few microns. This method would be suitable for the production of a high-grade chromium, provided an oxide-free chloride could be obtained.
Chromium nitride can also be prepared from chromium oxide by passim; ammonia over the oxide at 1,100° C. The reaction proceeds rapidly. Removal of the nitrogen at 1,400° C. in vacuo produces a metal as pure as that obtained from denitriding chromium nitride prepared from the chloride.
It is questionable whether the oxygen can be removed more completely by nitriding the oxide with a mixture of hydrogen and nitrogen or cracked ammonia than by direct hydrogen reduction. However, the reduction with hydrogen in a mixture with nitrogen permits the presence of larger quantities of moisture in the waste gases, and reduction proceeds faster because of the formation of the nitride. Traces of nitrogen that might remain behind in the metal after denitriding in vacuo at elevated temperature cause embrittlement.
Fusion Electrolysis
Hampe mentions the possibility of electrolyzing fused chromium chloride in a carrier salt. This process is mentioned frequently in the patent literature, but the details are not reported. Dolitskii claims that the most economical method of obtaining high-purity chromium is by fusion electrolysis.
Nees claims certain improvements, which consist of blowing hydrogen into the porous anode to improve the yield. The principal disadvantage in this type of reduction is the lack of stability of the chloride bath. Muskatt has shown that chromium chloride burns readily in air with liberation of chlorine . The use of a carrier salt, such as a mixture containing 60 percent potassium chloride and 40 percent sodium chloride, which melts at 650° C., permits conducting the operation at relatively low temperatures. It is questionable, however, whether the oxidation of the bath can be reduced in this way, as alkali chlorides are known to catalyze the oxidation of anhydrous plurivalent chlorides. Fusion electrolysis was investigated, therefore, to determine its industrial possibilities.
The fusion electrolysis experiments wore made in a graphite pot, which was used as the anode. It had an inner diameter of 6 inches and was 9 inches high. The graphite pot was placed inside an iron crucible, which was provided with an electrical connector. The fused salts were first conditioned with chlorine until they appeared oxide-free. The cathode, which was a 2-inch iron rod, was placed in the fused salt bath, and electrolysis was started. After about 1 hour, growth of dendrites caused short-circuiting with the anode. The cathode with the growth was removed and covered with dry, powdered sodium chloride to prevent oxidation of the chromium. After cooling, the material adhering to the cathode was removed and leached. The quantity of metal recovered gave an indication of the efficiency of the cell. The results of one typical experiment are shown in table 1. The average voltage was 10.5, and the amperage ranged from 750 to 1,275. The temperature ranged from 850° to 1,050° C.
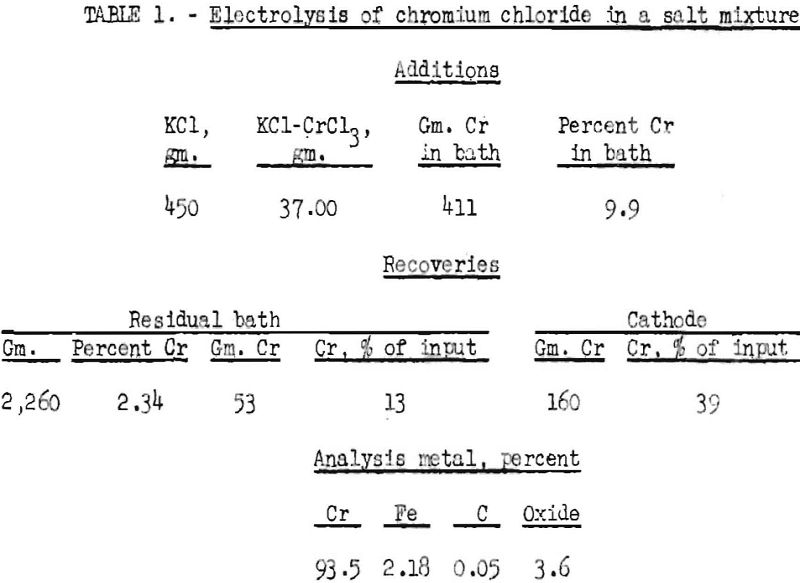
The recovery, based on the analysis, indicates a current efficiency of only 26 percent. The electrolysis of trivalent chromium salts, for instance, chromium trichloride, is certain to have a low current efficiency, because trivalent chromium chloride breaks down at the cathode to dichloride, by the reaction with the bulky chromium sponge that is deposited. On the other hand, divalent salt formed at the cathode moves to the anode by the turbulent action of the anodic chlorine and is chlorinated up to trivalent chloride. The reactions that take place at the anode and cathode can be formulated as follows:
Anode : 2 CrCl2 + Cl2 = 2 CrCl3
Cathode : 2 CrCl3 + Cr = 3 CrCl2
The use of a diaphragm would avoid these undesirable reactions by keeping the anodic salts in the trivalent and the cathodic salts in the divalent state. However, there is no material of construction known that would resist the combined action of the chlorides and the current.
It was not possible to establish a metal balance, because some of the chromium dropped off the cathode and was lost in the cell sludge. Some chromium was also entrapped in the cathode deposit in the form of chloride.
The chromium recovery, as metal, was only 39 percent, with 13 percent remaining after the experiment in the fused bath as chromium chloride. The purity of the metal was low, chromium oxide being the principal contaminant. Iron also was present in appreciable quantity. It was introduced through the action of chlorine and air on the cathode rod at the surface of the bath. This contamination can be avoided by shielding the cathode stem with a quartz tube.
If the surface of the bath is in contact with air, a constant reaction takes place between oxygen and moisture in the air and the salts at the bath surface. A closed cell and inert gas would prevent oxidation but would result in a complicated cell construction, and it would be difficult to remove the chromium deposited on the cathode. In an open cell the oxide formed at the bath surface, which is dispersed or dissolved in the bath, is decomposed at the anode by the action of carbon and chlorine. The balance that exists between the oxide introduced by oxidation and that eliminated by anodic action is very delicate. With a large exposed bath surface, or when operating the cell under conditions of high air humidity, only a small amount of badly oxidized chromium is deposited at the cathode. Some oxide is required to prevent anode effect in this type of high-current density cells, and a small quantity of the oxide, probably is soluble in the carrier salt.
It is rather difficult to remove the cathode, as the crystals adhere only loosely to the cathode rod or plate and may drop into the electrolyte when the cathode is removed from the bath. The sludge that forms on the bottom of the cell may build up and harden to such an extent that its removal might be difficult. The cathode sponge usually contains about 20 percent chromium, but it can be raised to at least 50 percent by pressing, as shown by one of the authors, who operated a similar cell for the preparation of iron. The vacuum method of removing the salts, as described later for the magnesium reduction of chromium chloride, might be unsuitable in this instance because of the presence of the chromium dichloride contained in the entrapped salts. Chromium dichloride has a low vapor pressure at 900° C., so that its removal in a vacuum at this temperature would be slow. Any oxide present In the entrapped electrolyte remains behind in the chromium sponge obtained in this operation.
The fusion electrolysis of’ chromium chloride would require considerable improvement before the process could be considered economically attractive. These improvements would include the repletion of the electrolyte with anhydrous, low oxygen-containing chloride, the reclaiming of the cathode salts by some dry method, so that no further leaching with water would be required, an efficient separation of the anode and cathode compartments to increase the current efficiency, and arrangements to remove the dangerous chromium chloride fumes.
Chromium from Amalgam
Chromium amalgam can be prepared easily by using mercury as the cathode in an aqueous cell with chromium sulfate or chromium chloride solutions. Chromium amalgam also can be obtained by reacting sodium amalgam with concentrated chromium-chloride solutions. Chromium can be extracted from the amalgam by evaporating the mercury, preferably in a vacuum. High-purity metal may be obtained in this manner, provided the amalgam is free from entrapped electrolyte. The pasty amalgam oxidizes superficially when handled in air, and the metal obtained may even be pyrophoric.
Recent German investigations appear to show promise for this method. Apparently, zinc can be produced by electrolysis with a mercury cathode on a commercial scale, and similar methods nay be applied to the production of manganese and chromium.
Dissociation of Chromium Iodide
Van Arkel claims that chromium can be made by the dissociation of its tri-iodide on a hot filament held at 1,100° C. while the vessel is heated to 800°-1,000° C. No information is given as to the malleability of the metal obtained. The operation mast take place in quartz vessels, and it is probable that this material is attacked by the iodide at the high operating temperature.
Chromium Oxide Reduction with Metals
In addition to silicon and aluminum, the metals calcium and magnesium also may be used for the reduction of chromium oxide. When the reduction step is carried out with magnesium, a black “lower oxide” is formed when the reaction product is leached with nitric acid to remove the magnesium oxide. Finely divided chromium is attacked by both water and nitric acid and forms a colloid, which passes through the filter. Two tests were made by calcium reduction of chromic oxide to determine the purity and malleability of metal obtained by this method. The results obtained are summarized in table 2.
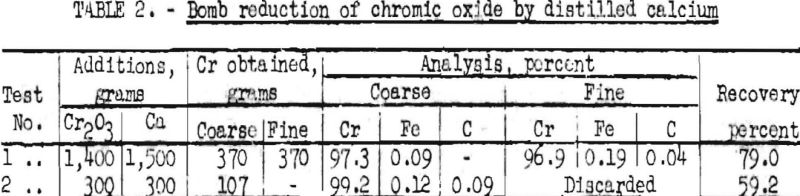
The coarse metal in test 1 averaged about 1/8 inch in diameter. After dissolution of individual pellets in hydrochloric acid, there remained a very small amount of dark-green oxide. In both tests, the sum of the constituents analyzed is not 100, so it may be assumed that some oxide was present as such and also in solid solution in the metal.
After degassing in vacuum at about 1,200° C., the products were fused in a beryllium oxide crucible in a helium and dry hydrogen atmosphere. The ingots obtained could be machined readily and had a hardness of Rockwell B-65. After sheathing the ingots in iron, they were heated to 800° C. It was possible to hot-roll the samples to some extent, but in both cases the metal was very brittle when cold. This observation confirms the previous observations of one of the authors.
The reduction of chromic oxide by calcium reaches an equilibrium depending on the relative weights of chromic oxide and calcium and also on the temperature and pressure obtained. To assure complete reduction, a low temperature, a high pressure, and a large excess of calcium must be used. In vacuo, chromium will reduce calcium oxide, and calcium metal is formed. The following equation rules this equilibrium;
2 Cr + 3 CaO = Cr2O3 + 3 Ca
The bomb method, therefore, is suitable for carrying out the reduction.
It was considered possible that the residual oxide, either dissolved in the chromium or free, could be reduced by a treatment with calcium hydride. Calcium hydride dissolves in fused calcium chloride, this solution resulting in an excellent liquid reducing agent. A sample of test 2 was treated in a hydrogen atmosphere with a fused bath of calcium chloride containing 20 percent calcium hydride. The product, after leaching and degassing, was fused in a vacuum in a beryllia crucible, but malleability was not improved.
According to Alexander, the substitution of calcium hydride for calcium as a reducing agent for chromium oxide improves the quality of the chromium obtained. As calcium hydride may be ground to a fine powder, it has an advantage over calcium metal shavings, as more intimate contact may be obtained with this powder, but more calcium oxide also is introduced into the reaction mixture when calcium hydride is used, as this compound decomposes more readily when in contact with damp air.
The degree of reduction of chromium oxide by hydrogen is ruled by the equilibrium pressure of the moisture in the waste gas. Once the reaction has reached the point where metal is formed, it slackens, because the oxide dissolved or entrapped in the metal must diffuse through the chromium particles to reach the surface. Therefore, to eliminate the last traces of oxygen in chromium by hydrogen reduction, dry hydrogen mast be used in large quantities, and a long reaction time is required.
It is dubious whether “nascent” hydrogen, which has, according to the literature, a life of only 30- seconds, can play a role in the reduction, which required a long time for completion; and the oxygen removal from the chromium must be attributed to the action of the calcium, which forms calcium oxide from chromium oxide and from water vapor. This is shown, also, when chromium metal is fused in the atomic hydrogen torch; malleability is not improved, nor is the metal deoxidized by the atomic hydrogen. Calcium hydride is very suitable for the elimination of the moisture contained in hydrogen, but the gas must be circulated slowly and for a long time over the hydride. A method for using other hydrides as a source for oxygen- and nitrogen-free hydrogen and as a circulation pump for this gas is given below.
Various methods were used in attempting to further deoxidize the calcium-reduced chromium metal. Some samples, after degassing at 1,200° C., were melted in beryllium oxide crucibles under helium, and snail amounts of zirconium, titanium, thorium, manganese , and aluminum were added. Also, a sample of chromium that had been reduced by calcium was distilled in a vacuum of less than 1 micron. The metal was condensed on a beryllium oxide cylinder and, after cooling, was remelted in a beryllium oxide crucible under helium. The remelted sample, as well as the samples with additions of zirconium, titanium, thorium, manganese, and aluminum, were all brittle when cold.
Adcock observed that when, chromium is heated to a high temperature in vacuo it is superficially covered with a greenish coat of oxide, possibly CrO. It seems that chromium metal is more volatile than its oxides, and most of the latter will stay behind when the metal is heated in vacuo to such temperature (for instance, 1,400° C.) that it evaporates. Deoxidation agents such as zirconium or beryllium when added to the chromium powder deoxidze the chromium at elevated temperature, as shown by the fact that briquets of chromium to which such deoxidizing agents have been added show a white oxide coat after heating in a vacuum of a few microns at 1,400° C. This permits adjusting the amount of the deoxidizing agent to the oxide present in the chromium, but the new oxide formed remains as inclusion in the metal, and the malleability is not improved.
The role played by oxygen as a contaminant of chromium metal could be studied with much better success, provided an accurate method were available for determining the oxide content by chemical analysis. The only feasible method appears to be hot extraction, in which fused high carbon-bearing iron is used as a solvent for the chromium, causing the oxygen to combine with the carbon and removing the gas by vacuum. Chlorination of the chromium-containing oxide by oxygen-free chlorine, which was reasonably successful for the determination of oxygen in zirconium, failed completely. A sample of carbon-free chromium-containing oxide, which after dissolution in hydrochloric acid left a dark-green residue amounting to 1 percent of the sample, was completely chlorinated at 900° C. and left no weighable residue. Apparently, chromium oxide reacted with the chlorine to form possibly chromyl chloride or some similar compound and was thus removed. This experiment also indicates some of the difficulties encountered in attempting to prepare an oxide-free chromium chloride by chlorination of the metal or of a chromium oxide-carbon mixture.
Calcium metal does not appear to be particularly attractive for the production of chromium from the oxide, considering its high cost and high atomic weight, and no cold-malleable metal has been made by calcium reduction.
Hydrogen Reduction of the Chlorides
The reduction of chromium chloride by hydrogen has been investigated thoroughly by Maier, who prepared a product containing 99.0 percent chromium by this method. A high hydrogen concentration (90 percent at 800° C.) must be maintained in the waste gases; consequently, large quantities of hydrogen must be recycled. As a result of recent progress in Germany, as reported by Murray et al the prospects of this process have improved because of the possibility of reclaiming the chlorine from the waste hydrogen chloride either by aqueous electrolysis or by the reaction of hydrogen chloride with air in a fused bath of potassium ferric chloride at 350° C. and collecting the chlorine in sulfur monochloride. German improvements of the Maier process, such as the reduction with hydrogen at a reduced pressure, as proposed by Schneider add more complications to this method. The hydrogen reduction of chromium chloride should be based on the production of pure, virtually oxide-free chloride direct from the ore. The chlorine and excess hydrogen obtained in the reduction of the chloride and combustion of the HCl are to be recycled. This method has not as yet progressed beyond the pilot-plant stage .
Chromium Chloride Reduction by Metals
According to the chlorine affinity table of the elements, it appears that chromium chlorides could readily be reduced by sodium, potassium, lithium, the alkaline-earth metals, beryllium, and also manganese. The alkali and
alkaline-earth metals appear to have an advantage, in that they do not alloy with chromium.
Sodium, when used under atmospheric pressure, produces a fine metal powder, but the chromium powder becomes colloidal during leaching. A more dense product is obtained in a bomb. The sodium chloride resulting from the reaction is volatile at 900° C. In a vacuum, so that the removal of this salt in vacuo is similar to the removal of magnesium chloride by this method.
Sodium metal is much more difficult to handle than magnesium, and finely divided sodium causes fire hazard. If these difficulties can be overcome, sodium may be used instead of magnesium as a reducing agent for chromium trichloride . It offers no price advantage, however.
Chromium chloride is reduced by manganese powder. Equilibria between solid metal powders and a fused bath are barely studied. A fused chromium-potassium-chloride mixture was reduced in a graphite crucible with powdered electrolytic manganese at 900° C. After leaching, a 95 percent chromium metal was obtained that still contained 2 percent manganese. This material was attacked by water during leaching. Vacuum evaporation, as carried out subsequent to magnesium reduction, as described above, would permit obtaining a high-grade chromium, but the high price and high atomic weight of manganese would not justify the use of this metal as a reducing metal.
Aluminum and silicon reduce chromium chloride, but chromium aluminide and silicide are formed in addition to the aluminum and silicon chlorides.
Zinc has been used by Wohler as a reducing agent for chromium chloride in a carrier salt. A zinc-magnesium alloy is more efficient than pure zinc. A zinc-chromium alloy is obtained, which, after removing the zinc in vacuo or after dissolving the ingot in nitric acid, yields a fairly pure chromium metal. The reaction has also been carried out more recently by Hanemann. The alloy obtained is low in chromium. It is pasty during the reduction and always entraps salts, which, if not free from oxide, will cause oxygen contamination in the metal. This method does not come in for serious consideration for the production of large quantities of chromium, and its value as to the possibility of making pure metal is somewhat doubtful.
Although there seems to be a considerable number of reducing agents for the chloride, the principal difficulty is to be found in the preparation of a chromium chloride free of oxide contamination. Chromium tetrachloride, which forms from chromium trichloride and chlorine above 600° C., is to a large extent dissociated below that temperature . It was discovered by Doerner and was isolated by Wartenberg.
Some properties of the trichloride may be stressed. The free chlorine that is evolved because of thermal dissociation when chromium trichloride is heated to elevated temperatures make it impossible to use this chloride in direct contact with any metal. This would limit the materials of construction of the equipment for handling this compound to either silica and silicon chloride.
Neglecting the fact that free chlorine is a product of the thermal dissociation of the two higher chlorides of chromium, all chromium chlorides react with iron, forming an iron-chromium alloy. Chromous chloride, for instance, reacts with iron as follows:
CrCl2 + 2 Fe → FeCl2 + FeCr
The iron-chromium alloy formed contains over 30 percent chromium. The reaction of chromium chlorides with iron is the basic reaction for one of the various “chromizing” processes and has been described by Becker and others. Similar reactions of the chromium chlorides may be expected with other iron-group metals, such as nickel, so that the choice of metallic materials of construction is very limited.
Special attention must be paid the oxygen content of the chloride. According to Muskat, chromium trichloride can be burned in air. The chlorine can be reclaimed, and the chromium is obtained as the oxide. Air must therefore be absent when condensing chromium chloride vapor. It is apparent that the production of oxygen-free chromium chlorides and their reaction in the vapor phase with a reducing agent such as magnesium presents far greater difficulties than those encountered by one of the authors in a similar process for making ductile zirconium.
A number of experiments were undertaken for the purpose of deoxidizing chromium chloride. Solutions of chromium chloride in a fused carrier salt can readily be made without using sublimed chromium chloride. Glatzel recommended the use of magnesium for the reduction of chromous or chromic chloride in a fused carrier salt such as potassium chloride. The mixture may be easily obtained by reducing potassium dichromate with ethyl alcohol in the presence of hydrochloric acid and drying. Glatzel reports that he obtained a chromium metal of 99 percent purity. These experiments were repeated, but the metal made analyzed only 96 percent chromium, 0.01 percent magnesium, and the balance mainly oxygen. The product was nearly black, which indicated the presence of a lower oxide.
Pokorny used the reaction given by Glatzel, but he separated the chromium by filtration from the fused magnesium and potassium chloride mixture. The problem of further oxide contamination still remains, as the product must be leached. The nitric acid and water treatment given the chromium attacks the finely divided chromium, and chromium oxide is formed. To eliminate the problem of further oxide contamination in the present work, the salts were removed by distillation at 900° C. and under a vacuum of 1 or 2 microns. This method of salt removal has proved very successful in the zirconium process. If the salts used are oxide-free and oxygen is avoided during the reduction, the resulting metal must be oxygen-free. Consequently, the chloride mixture must be deoxidized thoroughly before it is reduced to metal.
Deoxidation of anhydrous chromium trichloride is very difficult. As stated, CrCl3 is readily oxidized by air at elevated temperature according to the equation
4 CrCl3 + O2 = 2 Cr2O3 + 6 Cl2
This shows that hot chromium chloride should not be exposed to air.
Deoxidation may take place by subliming the CrCl2 in a current of chlorine. In this method, the temperature must be held above 700° C., and back reactions of the trichloride with the quartz walls of the container are possible. Chromium silicate and silicon chloride may form.
A better method for the deoxidation of chromium trichloride is based on the fact that the oxide can be reduced with carbon or carbon monoxide in the presence of excess chlorine. Carbon tetrachloride, sulfur chloride, and hydrochloric gas act similarly. The purification of the chromium trichloride by reduction of its oxide content is easy to achieve by dissolving the chloride in a bath of sodium potassium chloride fused in a pure graphite crucible, and by bubbling chlorine through the-melt in the absence of air. Possibly some Cr2O3 dissolves in the salt bath. The reaction takes place between graphite, chlorine, carbon, and the oxide solution. The reactions involved in this operation are
- Cr2O3 + 3 C + 2 Cl2 → 2 CrCl2 + 3 CO
- 2 CrCl2 + Cl2 → 2 CrCl3
- Cr2O3 +.3C + 4 CrCl3 → 6 CrCl2 + 3 CO
In reaction 1, two solids react with a gas, whereas in reaction 3 it is assumed that chromium oxide dispersed in the carrier salt reacts with carbon in the fused bath, and the chlorine for the reaction is supplied by the chromic chloride dissolved In the carrier salt to yield chromous chloride. The latter is reconditioned according to reaction 2.
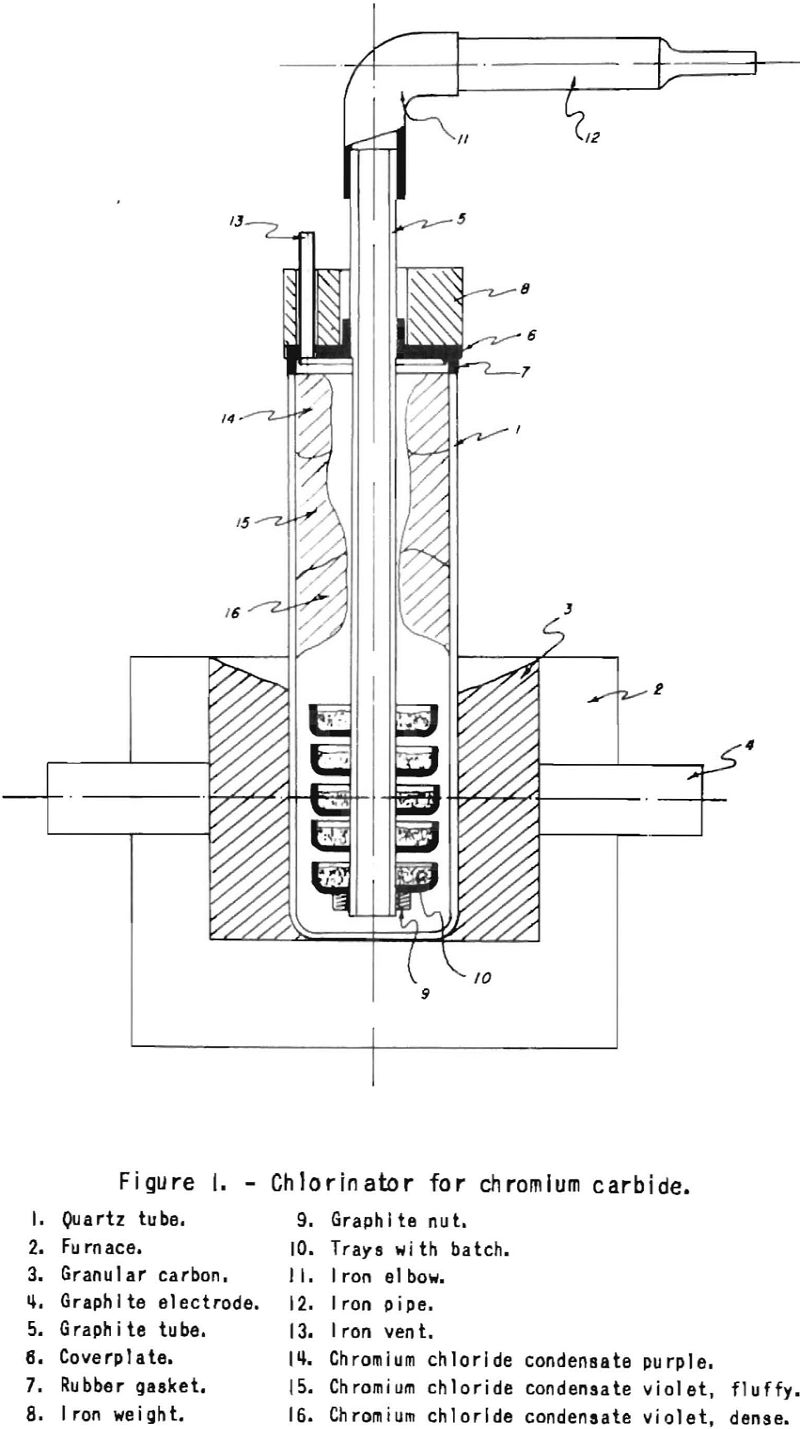
A typical instance of deoxidization of chromium chloride in a carrier salt will be described.
The starting material may be obtained either by reducing potassium chromate with ethyl alcohol in presence of hydrochloric acid and evaporating the solution, or by mixing crystallized CrCl2/CrCl3 and potassium chloride and heating to dryness . The dried salts may become green by hydrolysis of the chromium chloride and form Cr2O3. These salts are melted in a graphite crucible, which is inserted in a large quartz tube with sealed end. The arrangement is similar to that shown in figure 1. The tube is heated externally with granulated carbon, through which the current of a welding transformer is passed. Chlorine is bubbled through the bath at about 800° C. by means of a graphite tube. From time to time a sample is taken out with a graphite rod. When dissolved in water, this sample should not show a trace of green oxide. Chlorine is passed through the bath until all the dichloride present is transformed to trichloride. The saturation point of potassium chloride with chromium trichloride at 800° C. corresponds to about 55 weight percent CrCl2 in the mixture. Above this concentration, CrCl3 evaporates. The purification may take about 6 hours, after which the current of chlorine is replaced by nitrogen, until the bath freezes. The graphite crucible is broken up, and the lumps of salt are inspected as to their appearance. In general, the batch does not adhere to the wall, and there is no contamination with carbon.
Tho presence of 45 percent carrier salt is a drawback insofar as it reduces the reduction furnace capacity. In this regard, the use of oxide-free chromium trichloride would offer definite advantages.
Plain oxide-free chromium chloride can be obtained in the set-up shown in figure 1. Our starting material for the production of CrCl3 was iron-free chromium carbide made by the reduction of chromium oxide with pure graphite powder in the arc or high-frequency furnace. To produce oxide-free chromium trichloride from this carbide, specific conditions must prevail in the chlorinator. First, air must be absent to prevent oxidation of the chloride formed. In addition, excess chlorine must be present in each section of the chlorinator, as will be seen from the following properties of the chromium chlorides.
Chromium trichloride sublimes according to Maier between 600° and 800° C. in the presence of excess chlorine, which inhibits the thermal dissociation of this compound. Some unstable CrCl4 also is produced under these conditions. Chromium dichloride, which melts at 815° C and boils at 1,325° C., is a very deliquescent salt, in opposition to CrCl3, which does not dissolve in water. The thermal dissociation of trichloride to dichloride leads to difficulties, because the readily fusible dichloride impregnates the batch, and the action of the chlorine is inhibited. Chromium dichloride may also form by the reduction of trichloride with excess chromium carbide. Fused dichloride runs down to lower sections and obstructs the chlorinator. An excess of chlorine must therefore be maintained in each section of the chlorinator. This can to done on a small scale by using trays, as shown in figure 1, or on an industrial scale possibly in a Wedge-type furnace or rotary kiln. Any dichloride formed, for instance at the surface of the carbide, is chlorinated by excess chlorine up to the more volatile trichloride.
At temperatures above 800° C., quartz is attacked by chromium chloride and chromium silicate and silicon chloride form. Condensates of two different colors are obtained; that of the cooler -zone is fine and red-brown in color (fig. 1). This water-soluble product is contaminated with silica, which possibly originates from impregnation with SiCl4 produced by reaction of CrCl3 with the quartz in the hot zone. The hotter zone contains dense peach blossom chloride insoluble in water and suitable for reduction with magnesium. Peach-blossom chloride constitutes about 80 percent of the out pit.
Reduction of Chromium Chloride with Magnesium
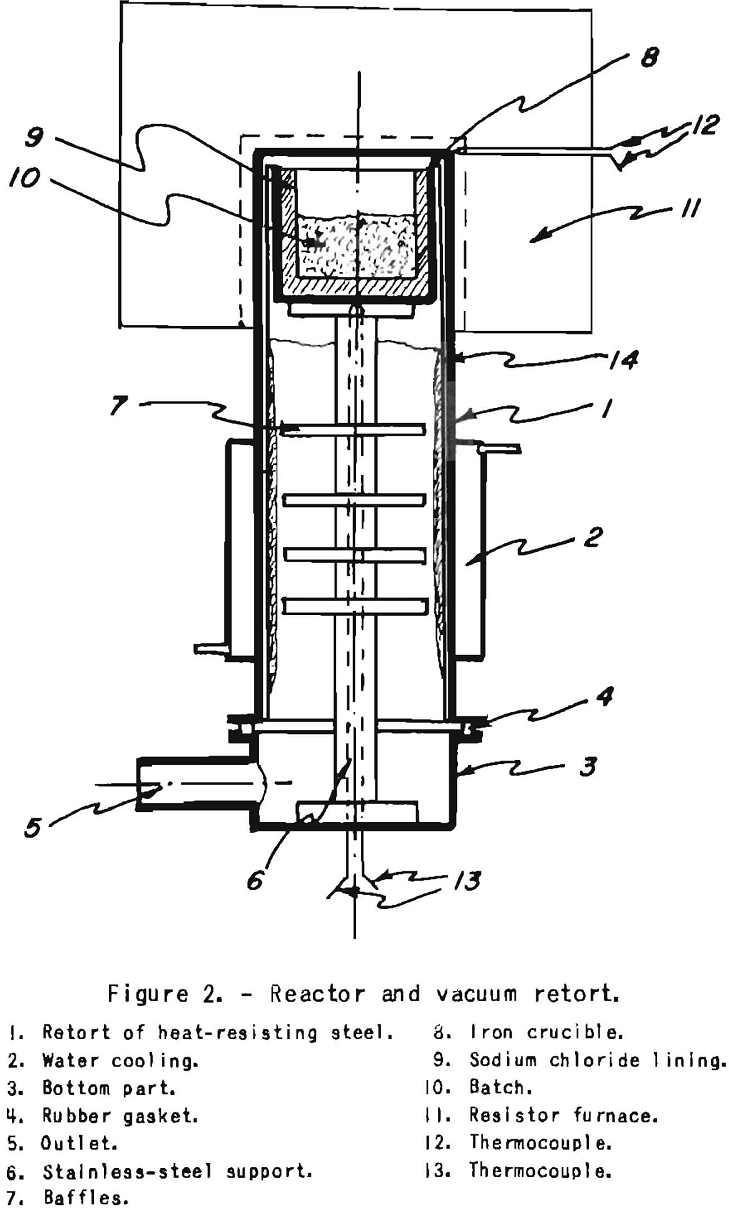
The deoxidizod. chromium chloride dissolved in a carrier salt, or plain trichloride may be reduced with magnesium by the Glatzel method. As shown above, any direct contact of chromium chloride with hot iron parts must be avoided. This can readily be done by lining the iron reaction crucible with sodium chloride powder that has been dried at about 500° C. The reaction between the magnesium and the chromium chloride takes place before the sodium chloride melts and before the trichloride dissociates appreciably. The fused bath, after the reaction, does not contain any free chromium chloride.
To start, the crucible lined with dry sodium chloride is filled with a loose mixture of sodium-chromium chloride or chromium trichloride and rough magnesium turnings. It is inserted in the retort shown in figure 2, which can be heated externally with an electric resistance furnace. The retort is heated slowly while a vacuum is maintained to draw off any moisture and residual gases. After reaching about 300° C., pure helium is admitted, and the temperature is brought up to about 800° C. Excess helium discharges into a rubber bag. A flash occurs at the onset of the reaction, usually at around 500° C. The maximum temperature is held for about 2 hours, after which the furnace is cooled to 500° C. A vacuum is then applied by means of a mechanical and diffusion pump arrangement, and the temperature is brought up age.in to about 850° C. to distill off the magnesium, sodium, and potassium chlorides in a vacuum of less than 1 micron. This requires about 4 hours, after which the furnace is shut off and cooled under a vacuum. The chromium recovery from the chloride is 100 percent. The salts could be melted out, as shown by Pokorny before evaporating in vacuo, by the use of a tap provided with a metal screen filter. Much time could thus be saved in salt evaporation.
The chromium powder obtained is a fine gray powder deposited in the form of a cake in the center of the crucible. It analyzes better than 99.4 percent chromium. It is free of iron but may contain a trace of chlorides. The spectrograph confirms these results. This chromium, which is very soft and plastic, is briquetted at a pressure of about 10 tons per square inch, degaased, and sintered at about 1,200° C. in a high vacuum, coined with 10 percent- cold reduction, and resintered under the same conditions, The Rockwell hardness, of sintered, coined, and resintered chromium ingots is usually up to B 65. The compacts can be cold-forged with 10 percent reduction, after which the Rockwell hardness increases to B 95. They can be rolled at 850° C.,
after sheathing in iron, with reductions of 10 percent per pass. At this temperature, chromium shows little stain when exposed to air. Chromium metal sheets can be bent at temperatures above 650° C. (fig. 6). The rolled chromium is brittle when cold. It resembles beryllium in this regard, which is malleable when hot but very brittle when cold. Tungsten, too, may be compared with chromium, insofar as it is brittle when cold; but thin tungsten wires or sheet show some malleability.
Purification of High-grade Chromium by Hydrogen Treatment
Rohn has shown that a very pure chromium metal may be prepared by reducing chromium oxide with large quantities of very dry hydrogen. The method described by him was used in a slightly modified form by the I. G. Farbenindustrie during the last war and has been described by Colcough. The reaction is carried out at 800°-900° C. in a vacuum of about 100 mm. mercury for 3 to 4 days. The process is of commercial interest.
The chromium powder obtained by reduction of the chloride with magnesium still contains traces of oxide, as shown by the fact that it is covered by a green film when heated in a high vacuum above 1,200° C. This oxide can be reduced by dry hydrogen at high temperature. Various means may be used to produce such dry hydrogen. Rohn dries the hydrogen with liquid air and circulates the gas by heating it with a hot filament in bottles provided with two sulfuric acid valves. This process does not permit eliminating nitrogen or other obnoxious oxygen-bearing gases.
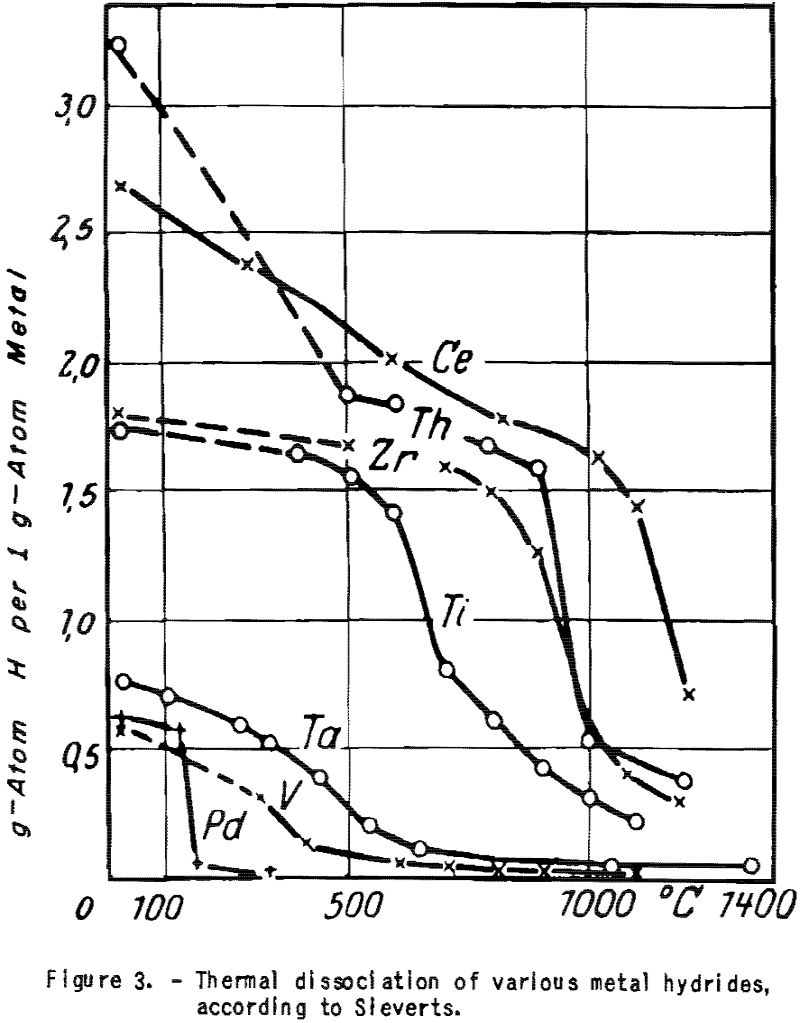
The hydrides of zirconium, thorium, titanium, and cerium appear to be excellent sources for dry and pure hydrogen. According to Sieverts (fig. 3), these hydrides lose about 50 percent of their hydrogen by thermal dissociation at the following temperatures:
Titanium 600°-700° C. Thorium 900°-1,000° C.
Zirconium 850°-950° C. Cerium 1,100°-1,200° C.
Calcium hydride can not be used for the present purpose as it dissociates with volatilization of calcium. As these metals also eliminate nitrogen, carbon monoxide, hydrocarbons, and hydrogen sulfide, they can be used as cleanser and circulation pump as well. Zirconium and titanium hydride can be obtained readily by reducing the oxides with magnesium in the presence of hydrogen.
The maximum permissible moisture content of the hydrogen used for reducing chromium oxide is, according to Koebel, 0.05 volume percent at 1,000° C., which corresponds to a dew point of 29° C. Liquid air and phosphorus pentoxide permit the moisture content of the hydrogen to be lowered veil below these limits, but the gas must be cooled before drying. At high reduction temperature, higher moisture content of the reacted hydrogen is tolerable. At higher temperature, however, increased sintering of the chromium powder obstructs the flow of hydrogen; also, the diffusion of the oxide to the metal surface, which is sluggish is important.
According to Martin, chromium dissolves 2 cc. of hydrogen per 100 grams of metal at 900° C. and 4.5 cc. at 1,150° C., and the hydrogen absorbed at high temperature is given off on cooling.
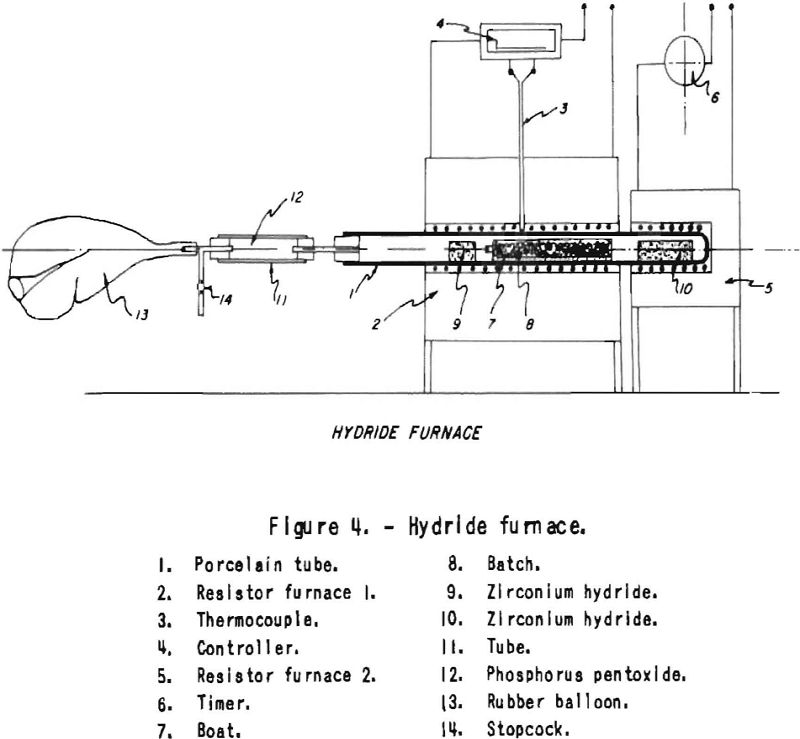
The equipment used for deoxidation with dry hydrogen made by way of hydride is shown in figure 4. It consists of a refractory tube, the middle portion of which is heated to 1,000° C. The sealed end of this tube contains a briquet of zirconium hydride, which is alternately heated by way of a timeslock switch within the limits of 500° to 1,000° C. Hydrogen is absorbed at low temperature; at high temperature it is desorbed. The flow of hydrogen is large enough to produce deoxidation of chromium powder within 4 days . The hydrogen coming from the hot hydride, after flowing over the sample and another hydride “safety” briquet put in front of the batch, is collected in a rubber bag. It is well known that oxygen diffuses through rubber. No trouble was encountered in our experiments from this source, as heavy-wall balloons were used. For safety, phosphorus pentoxide is used between the rubber bag and the combustion tube to take care of any humidity that may originate from the rubber balloon.
The bag may be replaced with another hydride briquet held at such a temperature that all the hydrogen driven off from the hot hydride would be absorbed by the low-temperature hydride-acting as gasometer. A small mercury-seal gasometer instead of the rubber balloon could take care of any differences between gas evolution or absorption of both hot or cold hydride briquets. When reducing plain oxides with this device, the bulk of the moisture could be eliminated with hot metal or alloy powder. This method of obtaining dry, nitrogen-free hydrogen and moving it at high temperature applies equally well to the sintering of chromium and chromium alloy powders or of such active metal powders as require very dry hydrogen for sintering for instance, Alnico magnet powders. This process may also be applied in the laboratory to dry and purify hydrogen, hydried being used as a gasometer. Figure 5 shows the set-up.
With a molybdenum resistor furnace higher temperatures could be obtained for the reduction. One of the main economical advantages in the use of hydride is that hydrogen may be dried without having to cool it to room temperature.

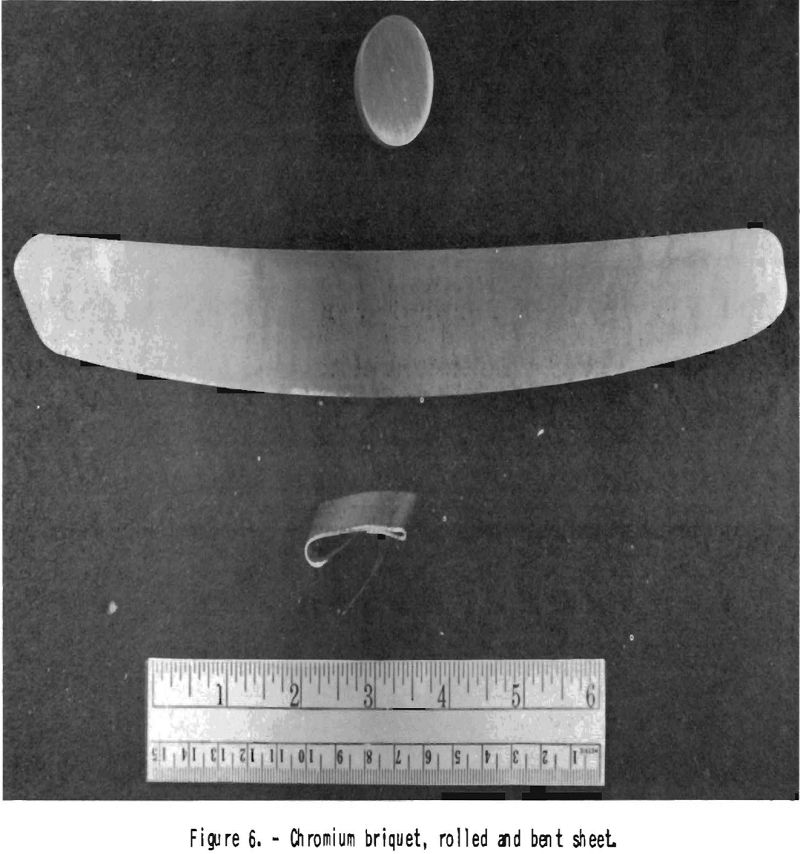
The chromium deoxidized in this way does not show a green coat when heated to 1,200° C. in a good vacuum. Its hardness is 5 Rockwell B units lower than that of the starting material. Sheets rolled from such material are shown in figure 6. A sample of oxide-contaminated chromium containing, by analysis, 97.0 percent Cr showed 99.6 percent Cr after 4 days treatment in dry hydrogen.
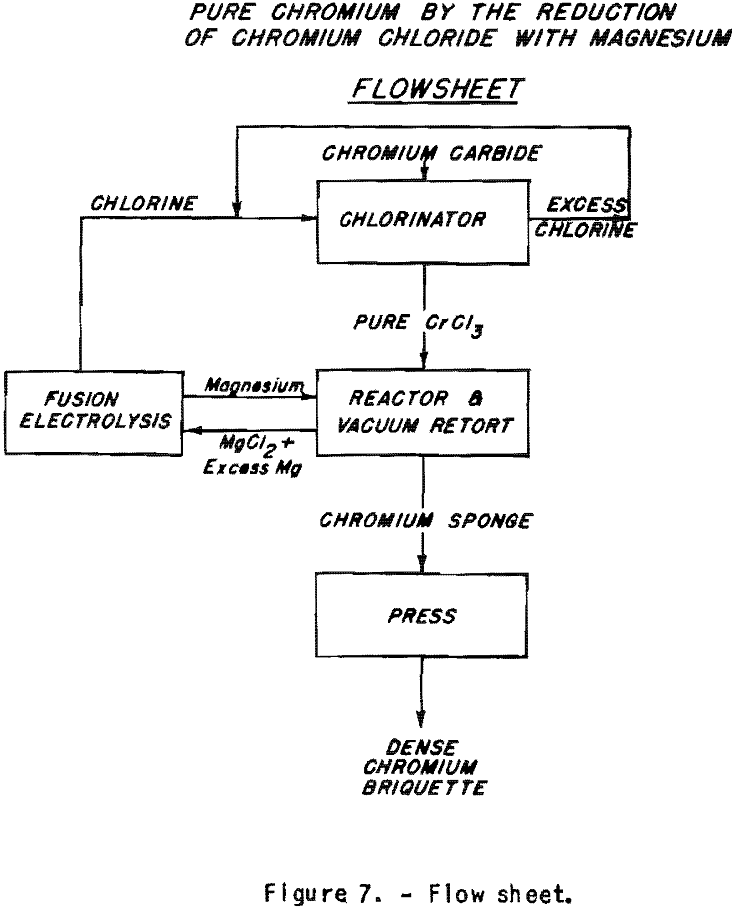
Production of Pure Chromium from Chloride on a Commercial Scale
Supposing that large quantities of chromium trichloride can be made cheaply, it would be possible to reduce this salt at lower cost than those resulting from the use of alumino-thermic methods for the reduction of the oxide. The flow sheet (fig. 7) shows how the magnesium and chlorine used could be recirculated. This would permit lowering the cost of the reducing agent by at least one-third, as the cost of production of the magnesium chloride is eliminated. Aluminum and magnesium are almost equivalent as to their market value related to their reducing power. Chromium carbide could be chlorinated directly to obtain the chloride. The chlorination of high-carbon ferrochrome permits elimination of most of the iron with virtually no chromium loss at temperature below 500° C. Starting from commercial high-carbon ferrochrome, carbon-free high-chromium alloys with iron could be made. The equipment for the reduction has been developed by the Bureau of Mines for the production of zirconium. It could be used as such for the reduction of chromium trichloride to sponge. The possibilities of this process are now being examined.
