Table of Contents
- Laboratory and Pilot-Plant Investigations
- Laboratory Tests on Production of Nickel Metal from Nickel Oxide
- Preliminary Reduction Tests
- Sagger Evaluation
- Operating Conditions
- Preliminary Melting Tests
- Pilot-Plant Production of Sponge Nickel
- Equipment and Facilities
- Supplies
- Preparation of Materials
- Procedure
- Operations
- Pilot-Plant Production of Nickel Ingots From Sponge Nickel
- Melting and Refining
- Costs
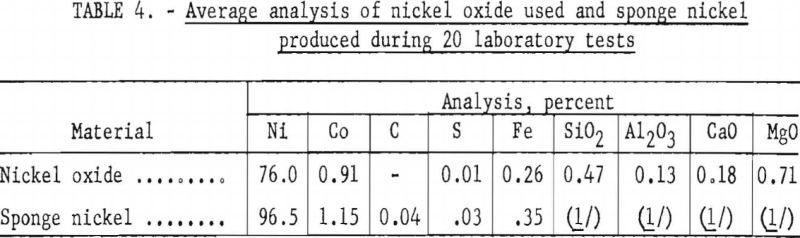
Sponge nickel can be produced by gaseous or solid-fuel reduction or a combination of both. Although pelletized nickel oxide was reduced in a laboratory rotary furnace, about 40 percent of the pellets abraded; magnetic separation was required to remove the fine sponge nickel from the charred coal and dolomite; hence, rotary-kiln operations were not considered further. Fines in the kiln would cause accretion or ringing. Furthermore, magnetically recovered sponge nickel would include iron and free carbon.
The use of nearly pure nickel oxide with a cobalt content low enough to meet specifications would produce a finished product without melting.
As equipment was not available, extrusion of nickel oxide as a charge material was not investigated; however, tests of such material would be advisable before large-scale operations are undertaken.
The binder used for pelletizing nickel oxide must be of such a nature that it does not increase the gangue or the impurities in the sponge nickel. Starch meets this specification, but the drying time (24 to 48 hours) presented a problem in pilot-plant operation and probably would present the same problem, involving a larger capital cost and tie up of nickel oxide in a large-scale operation. Near the end of the pilot-plant operations a satisfactory pellet was obtained by adding 0.5 percent bentonite and starch solution. Although some of these pellets were charged after a 1-hour drying period with no problems encountered, a longer drying time is probably desirable, and additional work on pelletization is needed.
The charging of nickel oxide, powder, and pellets, as received, as a layer between layers of anthracite, was tried when pelletizing could not keep up with kiln operation. This method is not recommended, as charred coal and dolomite adhered to the caked sponge nickel and part of the product tended to be unreduced because of variations in the thickness of the layers.
Although the open-hearth furnace could be used for melting sponge nickel, an electric-arc furnace is recommended. The latter would have an advantage of lower dust losses, increased recovery, and due to higher efficiency lower melting cost, provided power costs are not excessive.
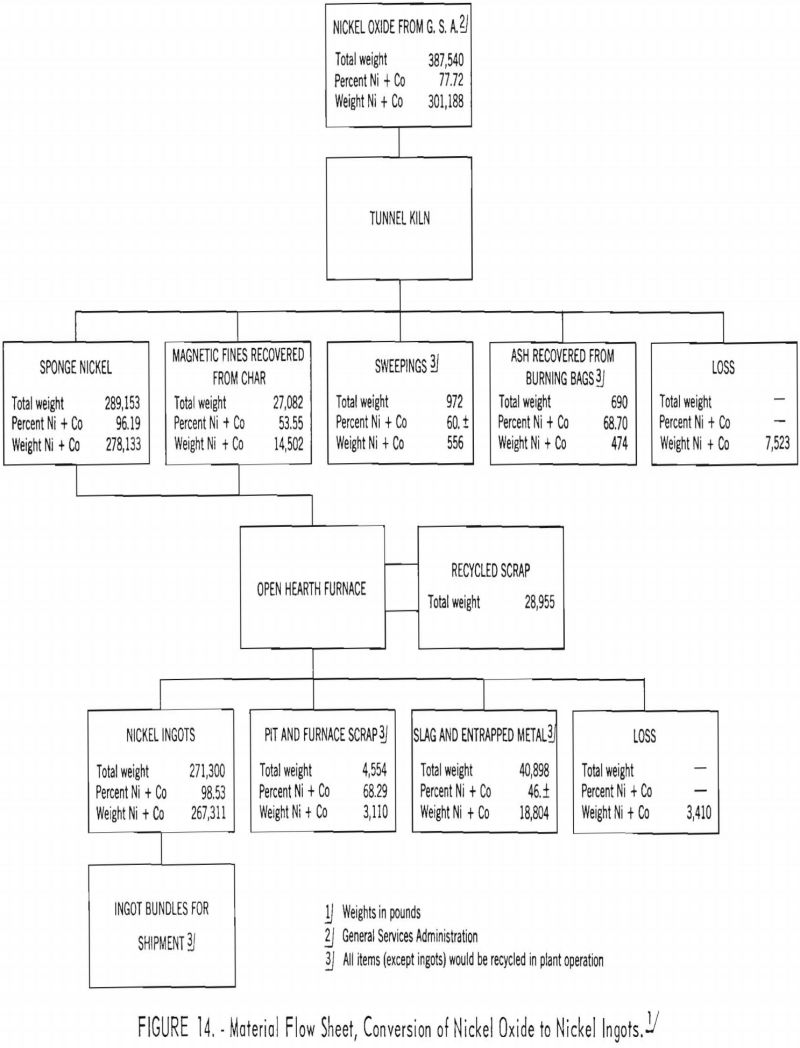
A two-slag system was used for melting the sponge nickel in the open-hearth furnace. The first slag (the meltdown slag) was removed with the metal temperature between 2,650° to 2,675° F., while the second (refining) slag was removed just before tapping with the metal temperature between 2,760° and 2,790° F. (Temperatures cited were taken with a noble-metal immersion thermocouple.) In the removal of the viscous meltdown slag, some metal was entrapped in the slag (see material flowsheet, fig. 14). This slag disintegrated however, after standing for a period and screening and magnetic separation were used to recover the metal for return to the furnace as scrap. Time did not permit completion of this recovery.

No difficulty was experienced in controlling sulfur content within specifications with use of the sponge nickel separated from the char and dolomite by screening; however, trouble was encountered in controlling sulfur when the fines recovered by magnetic separation were used. Also, an attempt to charge sponge to the furnace in nickel oxide bags failed because of the high sulfur content of the bags.
Blowing the metal bath with an oxygen lance was effective in removing carbon but had no noticeable effect in removing cobalt. Some cobalt was removed from the metal because the slag ratio of nickel to cobalt averaged about 30 : 1. Some well-controlled laboratory melts, using a neutral or acid slag, have shown a nickel-cobalt ratio of 10 : 1 in the slag.
The metal was deoxidized in the ladle, and calcium-silicon was more efficient than aluminum for this purpose.
From the results of laboratory tests and pilot operation, it is concluded that:
- Specification-grade nickel ingots can be produced by low-temperature reduction of Nicaro nickel oxide in a tunnel kiln and subsequent melting.
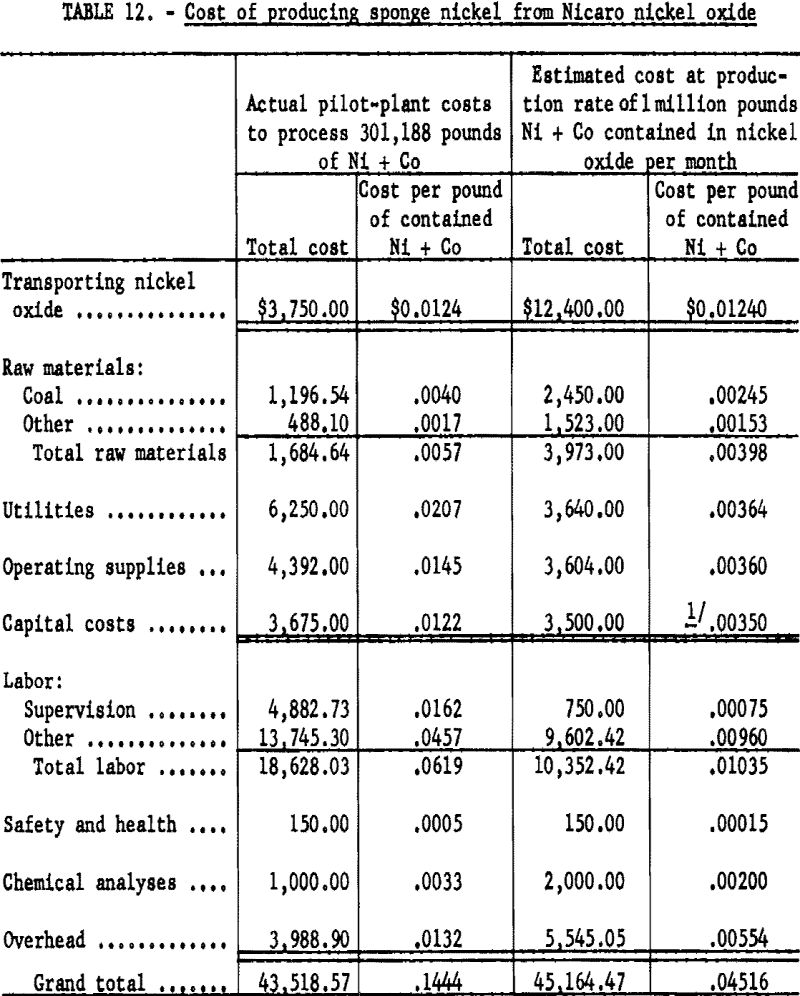
- Nicaro nickel oxide can be converted to sponge nickel at an estimated cost of $0.045 per pound of nickel plus cobalt, using ceramic saggers and a tunnel kiln in the Pittsburgh area at the assumed conversion rate per month of 1 million pounds of nickel plus cobalt contained in nickel oxide.
- Melting sponge nickel to ingots in an open-hearth or electric arc furnace at a cost of $0.03 per pound would increase the total conversion cost from nickel oxide to nickel ingots to $0.075. The total cost of conversion would be more than the value differential of the two products.
- To these costs must be added the equivalent cost of nickel losses, which would be very low in a well-designed and highly mechanized plant.
Laboratory tests verified that nickel oxide, in saggers and in the presence of anthracite fines and dolomite in the ratio of approximately 20:5:1, could be converted to sponge nickel when heated to approximately 1,900° F. for about 2 to 4 hours, depending on the size and density of the oxide charge. The tests also determined the type of saggers suitable for pilot-plant operations.
Pilot-plant operations for producing sponge nickel were conducted in a circular gas-fired tunnel kiln, with an effective length of 190 feet, at the plant of W. S. George Pottery Co., Canonsburg, Pa. Six carloads of nickel oxide weighing 387,540 pounds and containing 301,188 pounds of nickel plus cobalt were reduced to ingot metal.
The tunnel-kiln product, weighing 316,235 pounds and containing 292,635 pounds of nickel plus cobalt, was melted in a 4-ton basic open-hearth furnace. Ninety-seven heats were made; two slags were made to remove impurities, including part of the sulfur.
Nickel ingot metal containing 267,311 pounds of nickel plus cobalt was produced; the average analysis was Ni, 97.54 percent; Co, 1.00 percent; Fe, 0.73 percent; S, 0.059 percent; and C, 0.07 percent. Of the nickel and cobalt contained in the nickel oxide, 88.7 percent was recovered in the ingot metal, 7.7 percent was tied up in secondary products, and 3.6 percent was unaccounted for.
Based upon the pilot-plant operation data, the estimated cost of producing 1 million pounds of sponge nickel per month in the Pittsburgh area would be 4.5 cents per pound, including a transportation cost of 1.2 cents per pound but excluding handling losses.
The cost of melting the sponge metal to ingot metal in an electric arc furnace (melting capacity, 9,000 to 12,000 pounds) was estimated at 3 cents per pound, not including melting losses.
Introduction
Nickel is consumed by industry principally as metal and to a small extent as nickel oxide. Since production of the oxide is greater than requirements, part of the nickel oxide must be converted to nickel ingots for successful operation at the Nicaro plant.
Most of the nickel oxide is being sintered. A portion of this sinter is reduced to metal at Crum Lynne, Pa., in electric furnaces. Because the cost of sintering and electric furnace smelting to ingot exceeds the incremental value of the nickel oxide, other methods are sought that might lower the cost of conversion.
The Bureau of Mines was authorized by the GSA to conduct a pilot-plant operation using the Hoganas or sagger process for reducing Nicaro nickel oxide to sponge nickel. The nickel oxide contained about 1.5 percent of SiO2, Al2O3, CaO, and MgO; these impurities increased to 1.95 percent in the sponge nickel. The sponge nickel was melted to produce specification-grade nickel ingots by removing the gangue and part of the cobalt.
The project was carried out from January to September 1957. It was divided into three phases: (1) Laboratory testing (2) pilot-plant operation for production of sponge nickel, and (3) melting of sponge nickel in an experimental open-hearth furnace to produce specification-grade nickel ingots.
Laboratory and Pilot-Plant Investigations
Laboratory Tests on Production of Nickel Metal from Nickel Oxide
Preliminary Reduction Tests
A preliminary investigation by the Bureau of Mines in 1953 at its Pyrometallurgical Laboratory in Pittsburgh, Pa., on reducing nickel oxide in plumbago crucibles showed that low-sulfur, low-carbon sponge nickel could be produced from Nicaro oxide with carbon reductant. The purpose of further laboratory studies was to confirm results of the previous investigation and to determine optimum conditions for adaptation to pilot-plant operations. Two processes, the rotary kiln and the sagger-tunnel kiln methods, were tried.
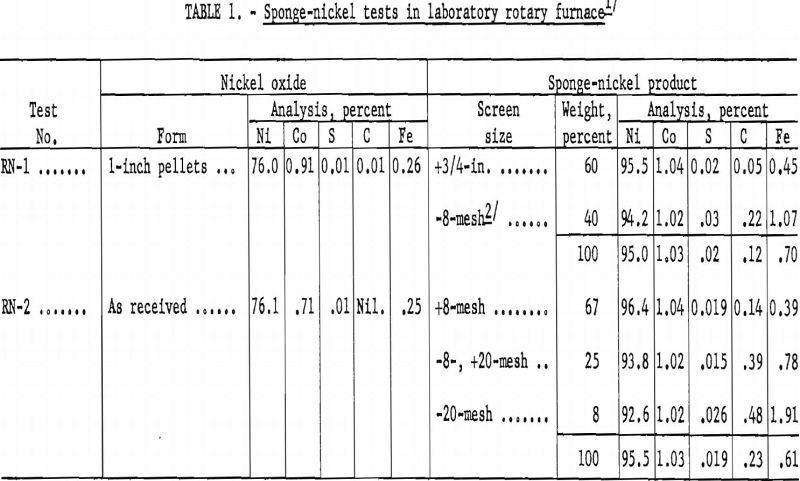
Although the sagger-tunnel kiln appeared to be the most suitable, it was deemed advisable to evaluate the rotary-kiln process; hence, two reduction tests were made in an 8-inch-diameter, externally heated rotary furnace. In the first test 1-inch hand-formed pellets were prepared from minus-20-mesh nickel oxide with a 2-percent starch solution as a binder. In the second test nickel oxide was used as received. The charge in both tests consisted of 4 pounds of nickel oxide, 3 pounds of anthracite fines, and 3/10 pound of sized (minus-8, plus-100-mesh) dolomite. Results of the tests are given in table 1.
From the results of the two rotary-kiln reduction tests the process appeared to have several disadvantages: A large percentage of the pellets would be abraded; the charred coal would have to be magnetically separated; the fine sponge nickel from the magnetic separation would have to be briquetted for furnace charging in melting to ingot metal; and the iron content in the sponge nickel would be increased. Because of these disadvantages, subsequent laboratory-scale work was concentrated on the sagger method for producing sponge nickel.
Preliminary investigations of several phases of the sagger process were made, including types and size of saggers, the form in which nickel oxide would be charged into the saggers, charge composition, and temperature and residence time required for complete reduction.
Sagger Evaluation
The following observation was made by other investigators on the use of pelletized material in sponge-iron production:
An unexpected difficulty was the tendency of the metallized glomerules to stick to the sagger wall and to weld together in a more or less compact mass within the sagger during reduction. They were extremely difficult to dislodge, and in many instances a sagger was broken in the unloading operation. Therefore, this problem was investigated by laboratory tests before pilot-plant tests were attempted.
As the durability of the sagger is a major factor in this process, a suitable sagger had to be proved by laboratory tests before being purchased for the pilot-plant operation.
The Ceramic Supply Co., a Division of Ferro Corp., Crooksville, Ohio, which had supplied saggers for a sponge-iron operation in Monterrey, Mexico, was invited to recommend the type, size, and composition of the sagger. The company recommended the use of its 144 mix and saggers that have an 11-½- inch outside diameter by 12-inch outside height, and furnished two saggers for test purposes.
Reduction tests were made on ceramic and calorized-steel (carbon steel impregnated with aluminum) saggers in a gas-fired muffle furnace. The ceramic sagger was approximately 12 inches in diameter and 6 inches in height and had a ½-inch thick-wall. The ceramic sagger used for laboratory tests had the same diameter but was approximately half the height of the saggers used in the subsequent tunnel-kiln experiments.
The calorized-steel sagger was 6 inches in height and 10-5/8-inches in outside diameter, and had a wall thickness of ¼ inch.
One thousand pounds of Nicaro nickel oxide was obtained for the laboratory sagger tests. A screen analysis showed that 28.5 percent of the nickel oxide, in the form of nodules, was plus-¼ inch; therefore, the plus-¼- inch fraction was removed by screening, and the minus-¼-inch fraction was pelletized before charging into the saggers.
Hand-formed pellets were tried first, and when no difficulty was experienced with sticking to the sagger wall or of pellets welding together, a laboratory pelletizer was built (fig. 1). Satisfactory pellets were produced by this pelletizer, using a 2-percent boiled-starch solution as a binder and air-drying the pellets 24 to 48 hours.
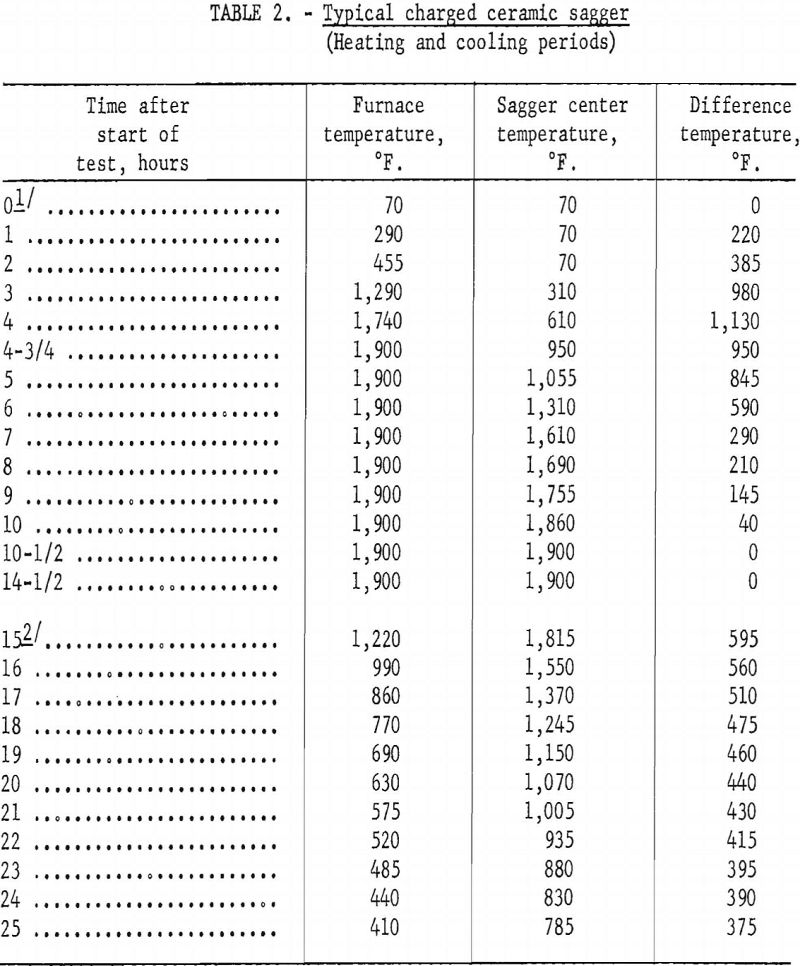
To avoid thermal shock to the ceramic sagger, heating and cooling were extended over 25 hours. In laboratory tests the ceramic sagger was charged into a cold, gas-fired furnace, heated as rapidly as possible to 1,900° F., and maintained at this temperature until the center of the charge had been at 1,900° F. for 2 to 4 hours. When reduction was completed, the gas was turned off and the furnace door opened as wide as possible to lower the temperature of the sagger and its contents to about 1,000° F. The sagger was then removed from the furnace and air-cooled until the temperature of the charge reached about 400° F.
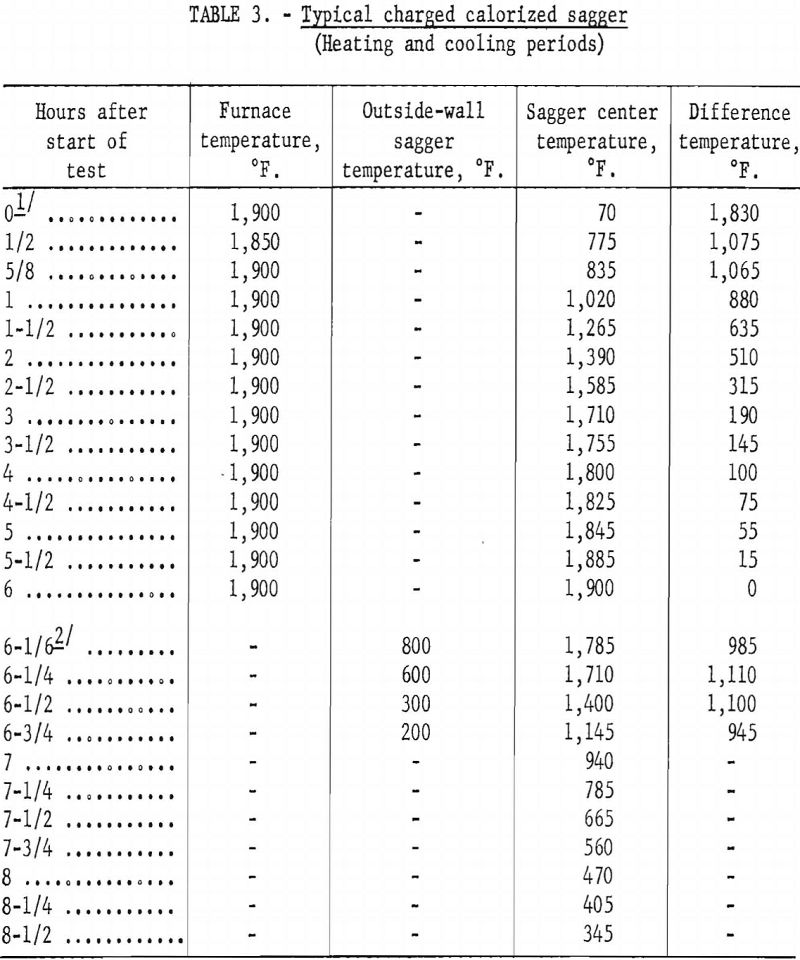
By contrast, the calorized-steel sagger was charged into the hot furnace at 1,900° F. and retained at temperature until the center of its charge had been at this temperature 2 to 4 hours. The sagger was then removed immediately from the furnace and air-cooled until the temperature of the charge reached about 400° F.
Tables 2 and 3 show typical heating and cooling cycles for fully charged ceramic and calorized-steel saggers. The ceramic sagger contained 30 pounds of ½-inch pellets prepared with starch binder, 7.5 pounds of anthracite fines, and 1.5 pounds of sized dolomite. The slightly smaller calorized-steel sagger contained 26 pounds of pellets, 6.5 pounds of anthracite fines, and 1.3 pounds of sized dolomite. Each sagger was covered with a circular lid, of the same material as the sagger, with a center hole through which a shielded chromel-alumel thermocouple was inserted into the center of the charge.
No attempt was made to evaluate metal vs. ceramic saggers in this short period. Depending on operations each has advantages and disadvantages. The metal sagger has the advantage that it can be handled without danger of breakage and is not affected by thermal shock. Laboratory tests showed that a metal sagger charged directly into the furnace at operating temperature,

removed from the furnace, and air-cooled would require a 9-hour time cycle, compared with a 25-hour time cycle for a ceramic sagger. The ceramic sagger has the advantage that it costs considerably less than a metal sagger; for example, the calorized-steel sagger costs about 10 times as much as a ceramic sagger. The ceramic sagger also has the advantage of withstanding high temperatures for prolonged periods.
Operating Conditions
Two factors had to be considered in determining the quantity of anthracite fines needed – the quantity required for reduction and the quantity required for separating the hot pellets to prevent their welding together. Previous work in 1953 indicated that consistent reduction of 99.5 to 100 percent could be attained using anthracite amounting to 15 percent of the weight of nickel oxide charged; however, tests showed that 25 percent anthracite fines was required when 12-inch-diameter ceramic saggers were used. With this quantity of coal reduction was complete, and the pellets screened individually from the charred coal. Since the size of the sagger used and the shape of the nickel oxide charged have a direct bearing on the quantity of coal used, the optimum quantity would have to be determined for each operation.
The results of a series of tests in which nickel oxide was reduced in both the metal and ceramic saggers showed a weight ratio of 20 parts of nickel oxide, 5 parts of anthracite fines, and 1 part of sized dolomite (minus-8, plus-100-mesh) was optimum when the charge was heated to 1,900° F. For complete reduction of the charge, it was necessary that the center of the sagger be at this temperature for 3 or 4 hours; the time depended on the size of the material being reduced and whether it was pelletized or nodulized. Table 4 shows the results of the reduction tests.
Preliminary Melting Tests
Since the specification for nickel ingot limits the cobalt content to 1 percent, one phase of the operation was to melt sponge nickel with a suitable refining slag to meet cobalt specifications. Results from preliminary melting tests conducted in a small carbon-resistance furnace using about 75-gram charges are given in table 5.
Several attempts to melt 2- and 3-pound samples of sponge nickel in the high-frequency 35-kv.-a induction furnace in silica crucibles, using a refining slag, resulted in crucible failures before equilibrium reaction had taken place. Successful melting tests were finally made by using a 3-½-inch-diameter crucible inside a larger silica crucible and packing the space between with the high-grade silica sand.
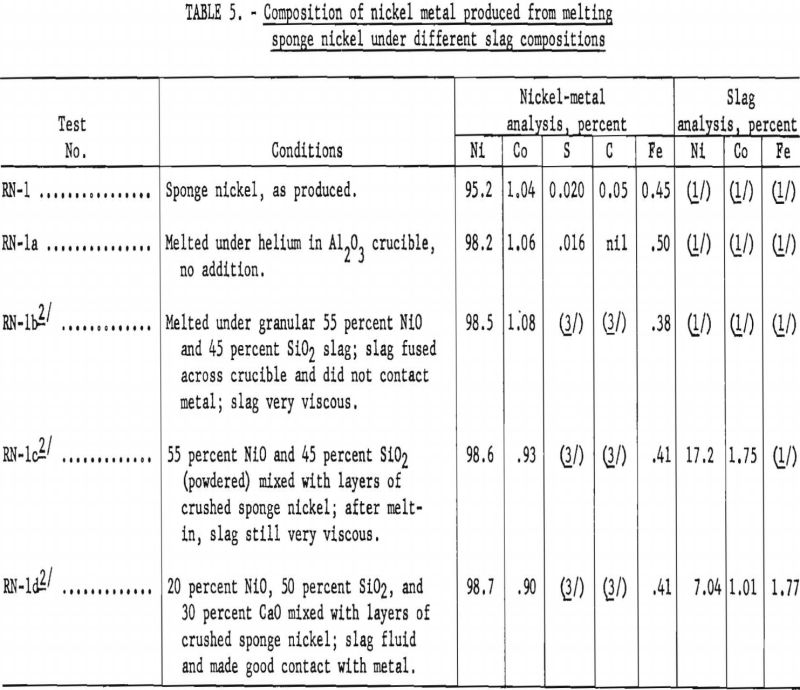
Using this procedure, a preliminary melting test with 2.25 pounds of sponge nickel, analyzing 96.06 percent nickel and 1.16 percent cobalt, was reacted with 0.40 pound of a slag-forming mixture consisting of 50 percent silica, 30 percent lime, and 20 percent nickel oxide. After the bath had become fluid, it was stirred with a silica rod and the melt poured into a metal mold. The nickel metal had the following analysis: Ni, 98.57 percent; Co, 0.92 percent; S, 0.058 percent; Fe, 0.40 percent; and C and Si, less than 0.01 percent. The resultant slag contained: Ni, 12.9 percent; Co, 1.37 percent; Fe, 2.59 percent; SiO2, 47.4 percent; Al2O3, 12.5 percent; CaO, 14.0 percent; and MgO 5.5 percent. The nickel metal recovered weighed 2.14 pounds, in which 97.6 percent of the nickel and 75.8 percent of the cobalt were recovered from the sponge-nickel charge.
Pilot-Plant Production of Sponge Nickel
The purpose of this phase of the project was to establish cost data on conversion of Nicaro nickel oxide to sponge nickel and to establish procedures that could be used as a basis for industrial production.
Equipment and Facilities
The main equipment rented and used at Canonsburg was a circular-tunnel brick kiln with a medial circumference of approximately 207 feet. A set of rails projecting through the kiln supported a train of 32 wheel-mounted flat-cars coupled to form an endless platform with rates of movement that could be varied from 0 to 19 feet per hour. Each car was 6 feet 5-½ inches in length and consisted of 12 rectangular lumnite blocks, 6 to each side, suitably supported, on which the saggers rested.
The permissible load limit on each flatcar was set nominally at 4 tons, which exceeded the experimental need of 5,200 pounds per car.
The kiln cars were loaded and unloaded at the open section of the kiln, which was 4-1/3 car lengths or approximately 28 feet long.
The kiln was fired by natural gas the air for combustion was supplied by two 16-ounce blowers set in series, with capacities of 975 and 675 cubic feet per minute. Fourteen burners were in the firing zone (7 on each side) and 6 burners in the preheat zone (3 on each side); which could be individually controlled as to input. Preheating of the saggers, other than by preheat burners, was controlled by a large fan at the entrance of the furnace, which pulled hot gases from the firing zone. Saggers coming from the exit of the kiln were cooled by a second large fan blowing air toward the firing zone. The working area for the operation was provided in front of the kiln and in the area encircled by the kiln. The storage bins were about 12 feet behind the kiln building, and the railroad siding was between the building and the bins. A door at the back of the kiln building gave access to the bins, and materials were brought along one side of the kiln to the working areas.
Other equipment used on the project were a small forklift, wheelbarrows, small bins for loading the saggers, and two roller conveyors. One of these conveyors was used for loading saggers and the other for unloading saggers from the kiln for screening.
Supplies
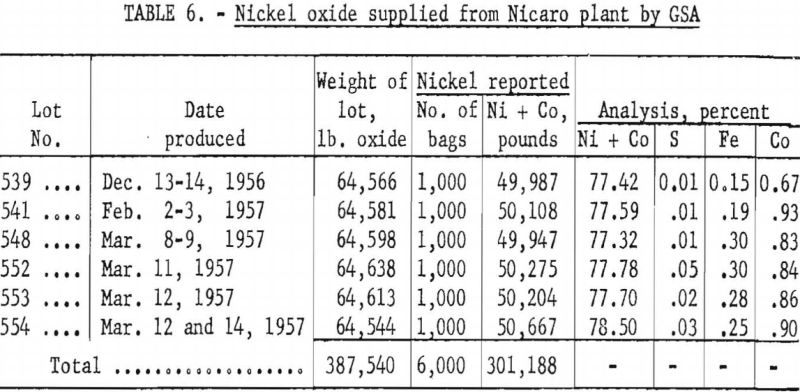
Six carloads containing 194 tons (6,000 bags) of nickel oxide was supplied by GSA from the Nicaro, Cuba, plant for this pilot-plant test. Table 6 gives the weights and analyses of the lots supplied.
Other raw materials needed for the operation were anthracite as a reducing reagent and dolomite as a reagent to control the sulfur content of the sponge nickel. A standard-size anthracite Buckwheat No. 4, which passes through a 3/32-inch hole, is retained on a 3/64-inch hole, and has a sulfur content of about 0.6 percent, was chosen for the operation. A commercial-size dolomite, minus-8, plus-100-mesh, which had previously shown excellent qualities for control of sulfur in sponge iron was chosen.
Ceramic saggers were used because of their low cost. The external dimensions of the saggers were 12 inches in height, and 11-5/8 inches in diameter, with a wall thickness of approximately ½ inch.
Operation of the tunnel kiln for producing sponge nickel was divided into two controllable divisions: (1) Control of the furnace temperature and throughput time and (2) preparation and use of the raw materials.
Control of the kiln was relatively simple. Once the burners in the tunnel kiln had been set, firing became automatic. The combustion air and gas were then controlled by an air-gas proportioning valve and mixing tube. The volume of air-gas mixture going to the burners was controlled by an automatic valve, actuated in response to temperature changes as indicated by two thermocouples in the firing zone of the kiln, which could be set to any predetermined temperature at the control board. During the operation it was not necessary to use any of the preheat burners and only 8 of the 14 burners in the firing zone. Once the kiln had reached temperature, the control point remained at plus or minus 5° of the set temperature, and other check points in the kiln remained at plus or minus 20°.
The fan at the entrance of the kiln, which pulled hot gases from the firing zone, was operated at full capacity during the entire operating period; however, the large fan on the exit end, which cooled the saggers so that they could be handled, never was operated over half of its maximum capacity, and no difficulty was experienced with cooling the saggers.
The only other control over the kiln was the speed at which the ram pushed the cars through the kiln. This rate could be controlled between 1 car in ½ hour to 1 car in 3 hours. The kiln was operated briefly at a speed of 1 car in 2 hours, but owing to material-handling problems the speed was slowed to 1 car in 2-3/4 hours.
Preparation of Materials
With the size of the anthracite and dolomite set, the size and shape of the nickel oxide had to be such that the sponge nickel could be separated from the charred coal and dolomite by screening.
Extruded or briquetted shapes were not tried because of the time limit for completing the operation; therefore, nodulized and pelletized nickel oxide, plus-¼-inch, was used. Since 35 percent of the nickel oxide had been nodulized to plus-¼-inch during calcining at Nicaro, removal of this material by screening minimized the pelletizing operation (fig. 1).
The original plan for the project was to screen the nickel oxide at 3/8-inch and pelletize the remainder before the start of kiln operations; however, because of delay in schedules the pelletizing had to be carried out at the same time as kiln operation. The screen size therefore was changed to ¼ inch to decrease the amount of material that had to be pelletized.
A two-deck open-type vibrating screen (¼-inch and 20-mesh) was used to screen the nickel oxide. In preparing the pellets the minus-¼-inch, plus- 20-mesh fraction and the minus-20-mesh fraction usually were fed to the pelletizer so that the minus-¼-inch, plus-20-mesh material acted as seeding for the pellets.
The open-type vibrator-screen arrangement caused high dust losses. An attempt to eliminate this step by pelletizing the nickel oxide as received directly from the bags and charging the pellets into the saggers without drying produced, upon firing, a conglomerate of sponge nickel, which retained considerable char and analyzed high in sulfur. This directly pelletized and wet charging of nickel pellets was stopped when the deleterious effect was first noticed, and the practice reverted to the original screening procedure, except that the minus-¼-inch material was not separated into two fractions.
The pelletizing operations required one man at least one turn per day. His work included making up a boiled-starch solution containing 1.6 to 1.8 pounds of starch to 10 gallons of water and transferring it to storage drums. The daily quantity of starch solution produced usually paced requirements, so that there was little spoilage, although this did occur in one or two instances when it was held several days at room temperature.
The drying time (24 to 48 hours) for the pellets, using a 2-percent boiled-starch solution as a binder, required more drying area than was available at the plant and prevented pelletization of adequate amounts of nickel oxide for kiln operation (fig. 2). Near completion of the run it was found that adding 0.5 percent western bentonite with the starch solution as a binder resulted in a more uniform pellet with a superior wet-handling strength, compared to the previous pellets. Pellets prepared with 0.5 percent bentonite and water alone had very little wet strength and crumbled upon drying.
Procedure
The basic procedures for producing the sponge nickel were determined in laboratory tests. Suitably mixed weight ratios of 20 parts of Nicaro nickel oxide pellets (½- to 1-inch diameter), 5 parts anthracite fines, and 1 part dolomite (minus-8, plus-100-mesh) fired in covered ceramic or metallic saggers in the temperature range of 1,950° to 2,000° F. for a period of 7 to 8 hours produced a high-quality sponge-nickel product. It analyzed 96.5 percent nickel and 0.03 percent sulfur or less (see table 4); the remaining constituents depended on the gangue entrapped in the nickel oxide before reduction.
In the pilot-plant operation it was not feasible to weigh accurately the nickel oxide and anthracite-dolomite mix used in each sagger because of the time factor involved; therefore, a volume-weight measurement was set up. A typical charging procedure by the operator, using ¼-inch nickel oxide pellets and a Buckwheat anthracite-dolomite mix was as follows: One-half scoop of anthracite-dolomite mix was scattered on the bottom of the sagger, followed by a mixture of nickel oxide and anthracite-dolomite. This procedure was repeated three times and a half scoop of anthracite-dolomite was used as a top layer to cover the nickel oxide (fig. 3).
The level of the charge material in each sagger was about 5 inches below the level of the sagger top, so that an air space was provided under each succeeding layer of saggers, which allowed good heat penetration and, consequently, more effective reduction of the nickel oxide.
Operations
Small bins containing the nickel oxide and anthracite-dolomite mixture were placed on a stand parallel to a roller conveyor (fig. 3). One man placed the saggers on the roller conveyor and loaded them, while a second man stacked them on the kiln car.
A car was loaded as follows: Three half-sections of tapered firebrick were laid on the lumnite block equidistant from each other with the narrow ends towards the center to form a tripod support for the saggers. Sturdy circular ceramic tripods, known as milkstools, were also used as sagger supports, but the supply was limited due to their relatively high cost. The purpose of using a tripod support, rather than placing the saggers directly on the lumnite car block, was to permit circulation of hot gases underneath the bottom sagger and, consequently, better heat penetration through the sagger. A loaded sagger was placed on the tripod support and leveled in an upright position, Saggers were stacked five high, each succeeding sagger functioning


as a cover for the lower one except for the top (No. 5) sagger, which was covered with a lid that is known in the pottery trade as a hiller. For a complete car six rows or columns five saggers high were placed along both sides of the length of each car. The space between the two rows was filled with dummy saggers. A layer of dummy saggers also was placed on top of the loaded saggers, since they lacked enough height to block off the kiln opening and prevent excessive loss of heat (fig. 4). This setting gave the usual 60 loaded saggers to a car.

For startup the kiln was loaded as follows: The first six cars were filled with dummy saggers and then each car was loaded with the 60 saggers. The cars were moved into the kiln progressively as they were loaded; meanwhile, the kiln temperature was being raised. When the dummy cars occupied the hot zone, car movement was stopped until the desired firing temperature of 1,965° F. was reached in this section of the kiln. The temperature was determined by 2 shielded platinum-platinum, 13-percent-rhodium thermocouples projected through the kiln wall and connected to recording instruments. Both thermocouples had been calibrated against a National Bureau of Standards calibrated thermocouple.
Six dummy cars were loaded by 3 p.m., April 20, 1957, and the 3 p.m. to 11 p.m. shift began filling saggers and loading the cars. At approximately 4 p.m. April 22, the 16th car entered the kiln, and movement of cars into the kiln was stopped. The kiln temperature was gradually raised until, on April 23 at 8 p.m., it was 1,200° F. Preparation of raw materials and loading of saggers continued, and on April 25 at 2 p.m., with the hot zone of the kiln at 1,915° F., movement of the car through the kiln was begun. By 5 p.m. the hot-zone temperature of the kiln had reached 1,965° F., the operating temperature for the test. The temperature remained steady thereafter until it was raised to 1,990° F. on May 17 at 8:30 a.m.; operations were carried on at that temperature for the remainder of the project.
Kiln operations were uninterrupted from April 25 to 9:30 a.m., May 26, when failure of an improvised sagger support caused a wreck in the kiln, which necessitated cooling the kiln to remove the debris. The kiln temperature was up to 1,975° F. at 5:30 a.m. June 2, at which time full-scale operations were resumed. Car movement was stopped June 5 at 8:05 p.m. as the last charged car moved out of the hot zone. Cooling of the kiln was started by lowering the temperature at the rate of 50° F. per hour.
In the first cycle of operation only plus-¼-inch nickel oxide was used, but as the operation became stabilized various forms of the nickel oxide were charged. Nickel oxide was charged (1) as oxide directly from the bags without screening; (2) as undried pellets; (3) as minus-¼-inch, plus-20-mesh oxide; (4) as minus-20-mesh oxide; and (5) as pellets made from “as-received” oxide.
As the operation progressed and it became difficult to pelletize rapidly enough to use all the nickel oxide fines being produced, a variation in charging the saggers was effected. A layer of anthracite-dolomite was placed on the bottom of the saggers, followed by two scoops of nickel oxide fines, one or two scoops of anthracite-dolomite, and two more scoops of nickel oxide fines; then all was covered with a scoop of anthracite-dolomite to give a “pancake” or sandwich layer. Some of the first pancake sponge nickel produced in this manner had green centers, which indicated unreduced nickel oxide, but this condition was corrected by increasing the quantity of coal in the charge and raising the operating temperature from 1,965° to 1,990° F. Although this meant changing 2 variables almost simultaneously, time did not permit reliance on merely increasing the coal charge, since the average retention time in the kiln for a charged sagger exceeded 3 days.
The saggers coming from the exit of the kiln were removed and placed on a roller conveyor for screening (fig. 5).
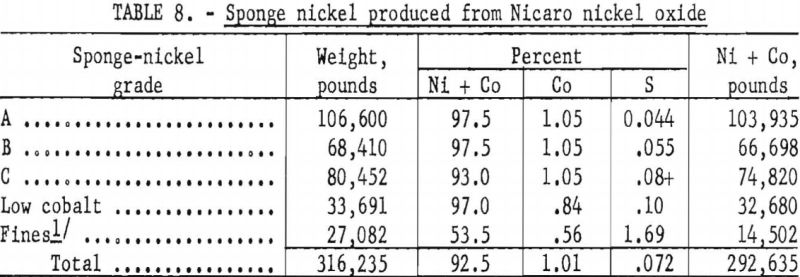
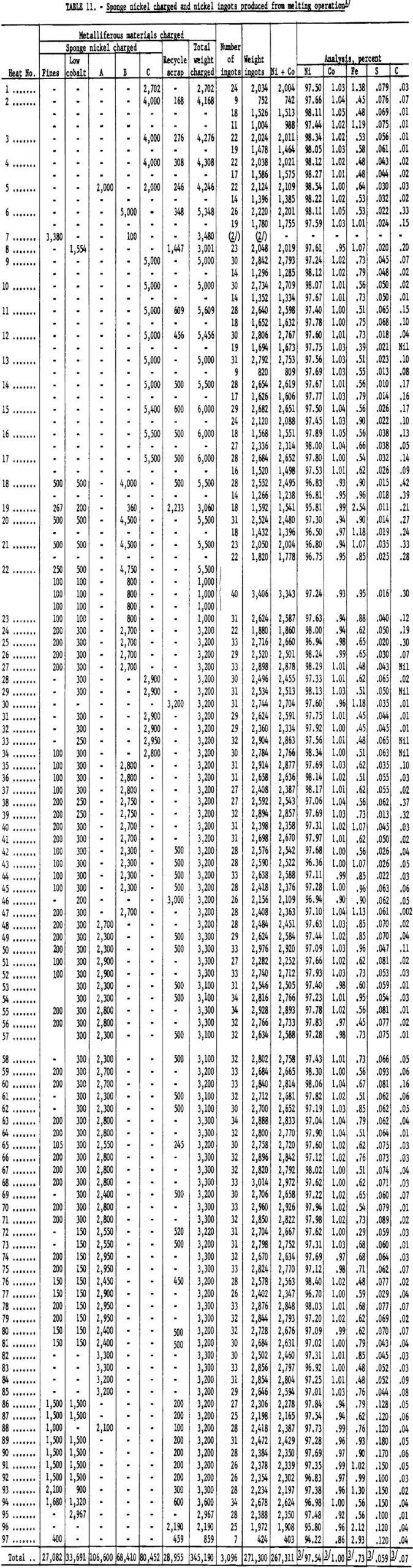
The sponge nickel was separated smoothly by screening with loose sponge-nickel pellets produced in accordance with previously tested laboratory methods from either plus-¼-inch screened nickel oxide or air-dried pellets made with a 2-percent starch solution. The loose, nonadhering char products dropped freely through the 8-mesh screen, leaving clean sponge nickel, which was designated Grade A. This nickel metal, amounting to 34 percent of the sponge produced, averaged approximately 96.5 percent Ni and 0.044 percent S.
The pancake-shaped sponge nickel (fig. 6), formed from layers of either minus-¼-inch, plus-20-mesh nickel oxide or minus-20-mesh nickel oxide or mixtures of both, sandwiched between the anthracite-dolomite mix, was harder to handle in the screening operation, as char products were embedded in the surface. Vigorous agitation of the shaker screen resulted in fine particles of sponge nickel breaking off and passing through with the char products. Nearly half of the sponge nickel produced was of the pancake type designated Grades B and C. Grade B was not of as good quality as Grade A; it averaged 96.5 percent nickel and approximately 0.055 percent sulfur. Pancake material containing more than 0o06 percent sulfur was called Grade C.
The third sponge-nickel product, Grade C, had three sources: Directly pelletized, unscreened nickel oxide, charged while still wet into saggers in


an effort to avoid losses of dust by screening; recharged pancake-shaped sponge nickel, which had green centers because of incomplete reduction; and sponge nickel either granular or pancake-shaped, which was produced with high-sulfur. Nut-size anthracite when anthracite fines were not available. This product contained 92 percent nickel and 0.08 percent sulfur or higher.
The “low-cobalt”-grade sponge nickel (see table 8) was produced by pelletizing and reducing part of the nickel oxide (lot 539, table 6), which was low in cobalt, as received. The “fines” grade was obtained by magnetic recovery from char.
Once it had been determined that the nickel oxide was fully reduced and the sulfur controlled, only those changes in the operating procedure that were necessary to continue production of a good-grade sponge nickel were made, such as sagger-charging procedures. With the limited time available for the operation and the large tieup of material within the tunnel kiln, experimental changes could not be risked. With a ram push of 1 car in 2-¾ hours – the maximum speed at which the kiln could be operated, owing to material-handling problems – 70-¾ hours was required for a sagger to pass through the kiln.
Except for a few samples to determine the degree of reduction, the samples had no control value and were used only for maintaining a record. After the furnace operations were established, a system of sampling was set up whereby every 25th sagger column was removed to furnish a sample for analysis. Table 7 shows the average analysis of the control samples.

The sponge nickel produced at Canonsburg, Pa., was far from uniform in shape and analysis and was divided into five grades for melting at Bruceton (see table 8). Plant operations produced a product virtually uniform in physical size and analysis, which would adapt to a routine melting operation once the procedure was established.
Pilot-Plant Production of Nickel Ingots From Sponge Nickel
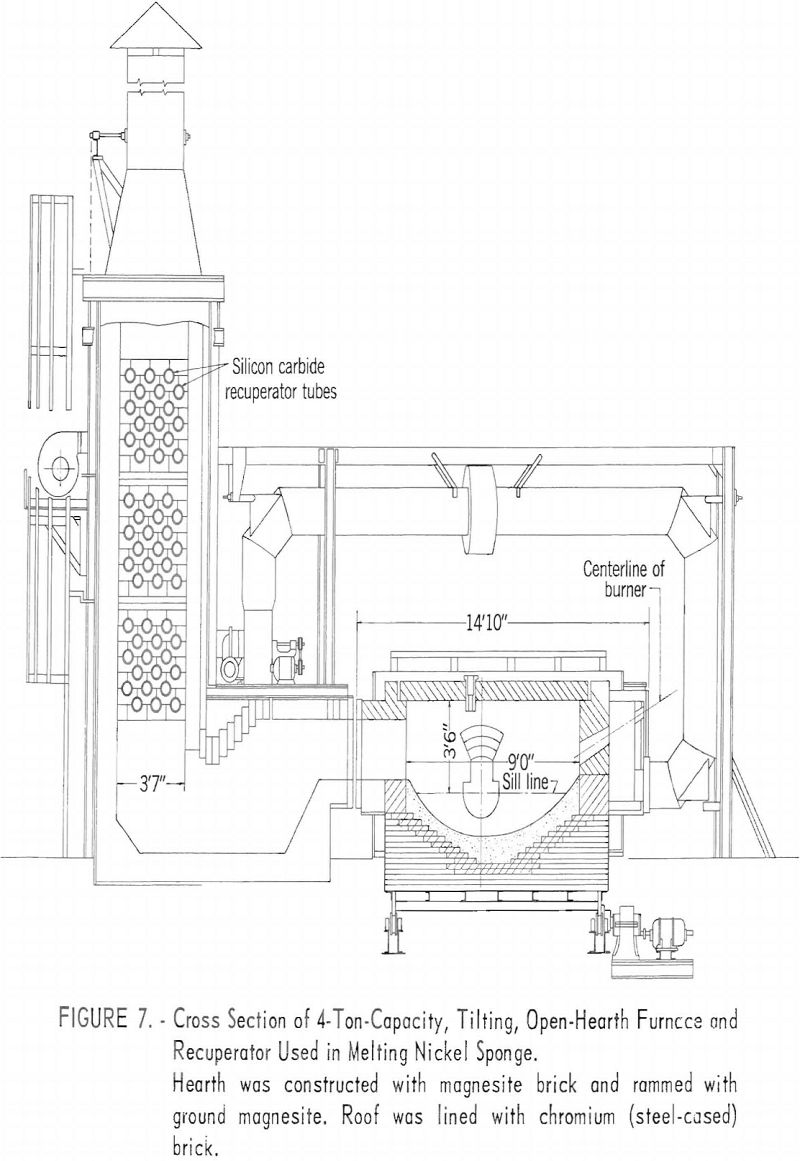
The sponge-nickel melting operation was conducted in a 4-ton, tilting, open-hearth furnace at the Bureau of Mines Bruceton, Pa., Station. A sectional view of the furnace and recuperator is shown in figure 7.
The furnace was fired with natural gas and preheated air from the recuperator. Additional heat in the furnace was obtained by oxygen enrichment of the combustion air. The various melting operations are shown in figures 8-13.
Melting and Refining
The first procedure for melting involved charging into the furnace 5,000 to 5,500 pounds of sponge nickel, about 500 pounds of nickel scrap from previous heats, and 80 pounds of pebble lime. After meltdown was begun, 20 to 50 pounds of fluorspar and lime was added to obtain a slag that could be raked from the furnace. Upon completion of the meltdown, samples of the slag and metal were taken, and the first slag was removed from the furnace. Flux for a second refining slag was provided by charging into the furnace a mixture of 60 pounds of pebble lime, 20 pounds of fluorspar, 50 pounds of sand, and 20 to 25 pounds of nickel oxide. After the slag had become fluid, samples of metal and slag were again taken. The metal bath was then blown with oxygen for a short period (usually 2 minutes), and another sample of metal was taken. When the immersion thermocouple indicated the metal temperature to be between 2,750° and 2,800° F. the slag was removed, and about 60 percent of the metal was tapped into the ladle and poured into molds. After the molds had been stripped, the remainder of the metal in the furnace was tapped.
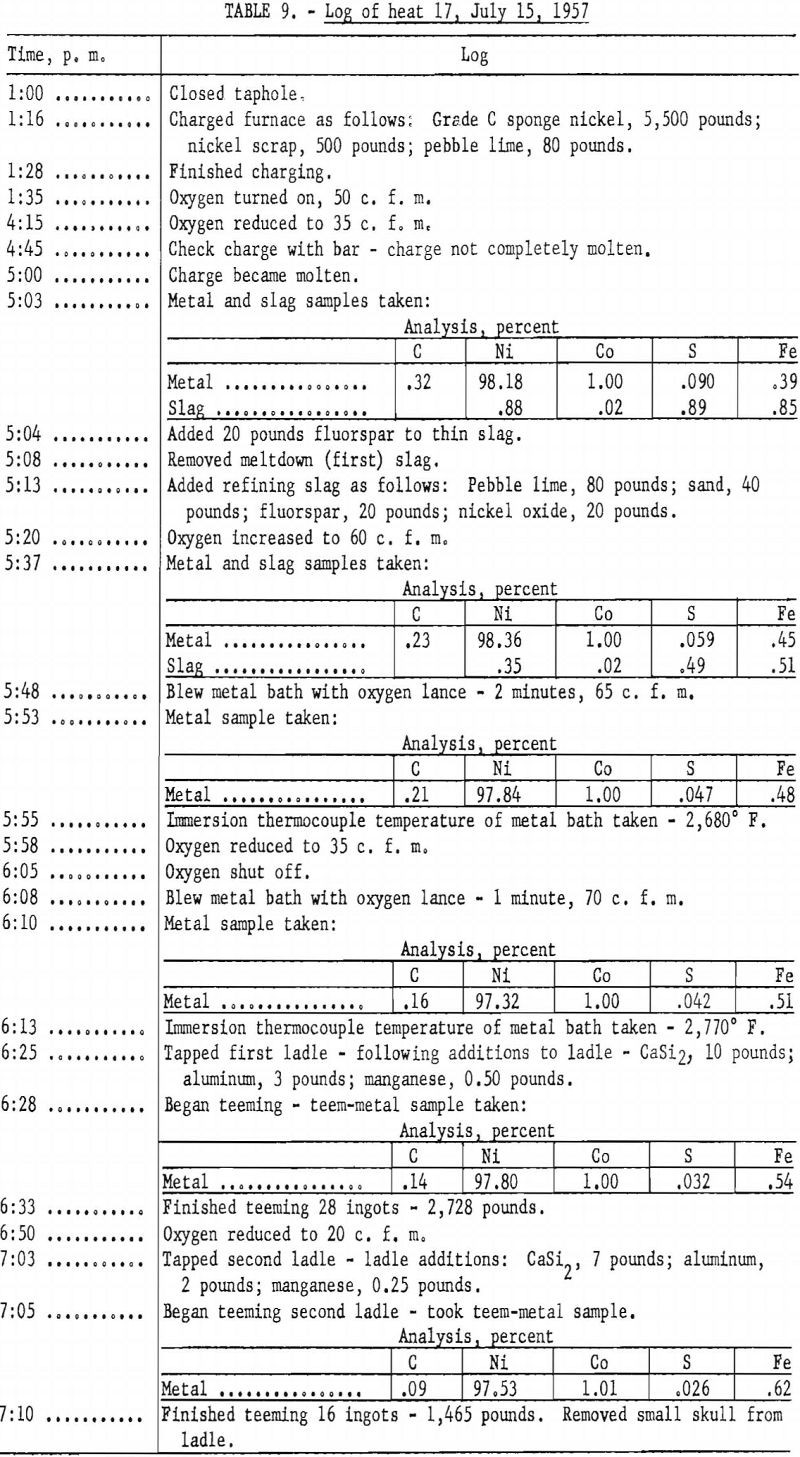
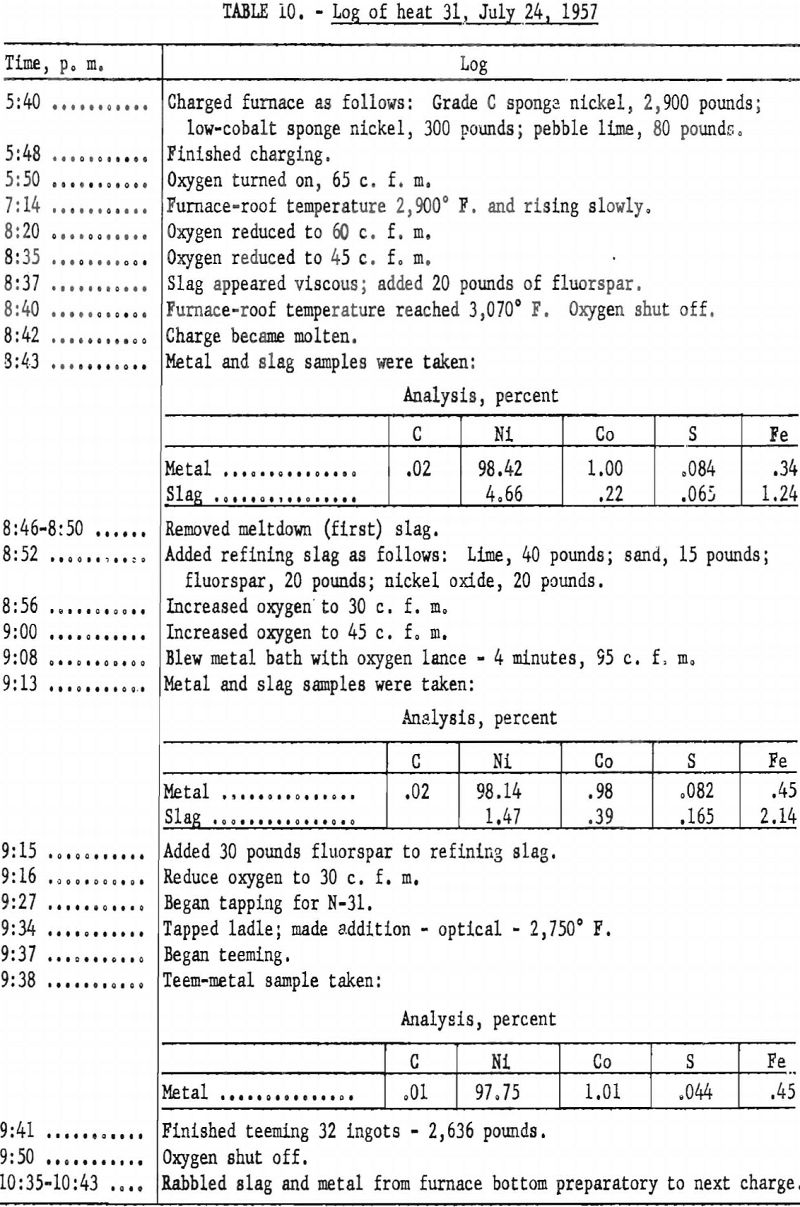
The log of heat 17 (table 9) shows working of a typical heat, using the above procedure.
With two taps per heat, the excessive lancing required to open the tap-hole increased the iron content of the nickel ingot on the second tap, which in many instances exceeded the specification limit. To overcome this difficulty the size of the charge was reduced to 3,200 pounds, or about 40 percent of furnace capacity, to permit a single pouring of all the molten metal into ladle and molds.
A log of heat 31 (table 10) shows the working of a typical heat using the smaller charge.
Ninety-seven heats were made in melting the sponge nickel produced at the Canonsburg, Pa., operation. A temperature of 2,750° to 2,800° F. was considered adequate to complete deoxidation and pouring of the ingots. Higher metal temperature could have been obtained by maintaining the metal in the furnace for a longer period. Table 11 lists the amounts and analyses of metal tapped from the 97 heats.

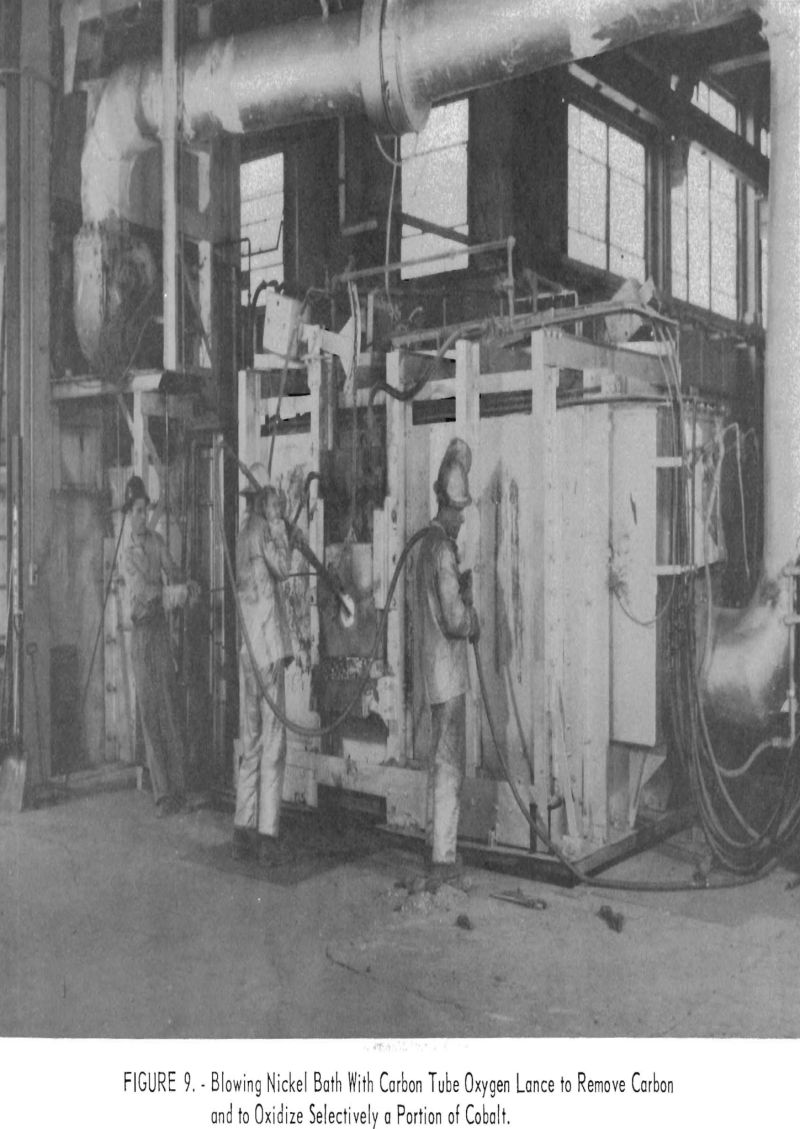
In melting the sponge nickel no reducing agent was needed. Melting the sponge-nickel cakes, which contained entrapped coal, yielded metal with carbon contents higher than specifications. Carbon was blown from this metal with oxygen, which also increased the temperature of the metal. It was expected that some selective oxidation of cobalt would occur when it was brought to or under the specified 1 percent. Although information was not conclusive on this point, all melts were blown with oxygen to increase metal temperature and possibly to remove cobalt from the metal. Oxygen blowing achieved good removal of carbon but no substantial reduction in cobalt or iron.
To avoid oxygen pickup when oxygen was used for blowing, a control sample with carbon content at meltdown should be used to determine the blowing time. As no carbon analysis was available during the heat, overblowing was necessary, and some trouble was encountered in deoxidizing the metal in the ladle.



The metal was deoxidized in the ladle. In the first heats aluminum was used for deoxidation; however, it was found that the efficiency of the aluminum was low, because the aluminum burned instead of going into solution with the metal. Calcium silicon was tried with good results and efficiencies, and a combination of calcium-silicon and aluminum was used in the remaining heats. Calcium-silicon could have been used alone, but it was necessary to substitute some aluminum, since the supply was limited.
Sulfur was readily removed from the sponge-nickel pellets. With the 2-slag operation, the melting metal (containing as high as 0.10 percent sulfur) could be lowered to 0.04 percent or less; however, the melting of magnetic fines containing as high as 1.5 percent sulfur presented a serious sulfur-removal problem.
The only cobalt removal was through a preference of the slag for cobalt over nickel, and the ratio of nickel to cobalt in the slag averaged about 30:1 in comparison to about 100:1 in the metal.
Costs
Table 12 gives a breakdown of the actual costs of processing 6 carloads of nickel oxide containing 301,188 pounds of nickel plus cobalt and an estimated cost of processing nickel oxide containing 1 million pounds of nickel plus cobalt per month. The biggest problems encountered in pilot-plant operation were in manual handling of raw materials and controlling dust from screening. The process can be largely mechanized to overcome the difficulties encountered in handling materials.
Dust losses could be reduced appreciably with a dust-collection system that included a small baghouse, but the problem would be virtually eliminated if the oxide was screened at Nicaro to furnish coarse material.
On the bases that the plant would be mechanized wherever possible and that wage rates would be the same as those of Bureau of Mines, wage-board employees, the following assumptions were made to arrive at the estimated cost:
- Plant would be mechanized, except for loading, placing, and removing saggers; a dust-collection system, including a small bag-house would be used.
- Estimated cost of tunnel kiln, building, and accessories would be $200,000.
(a) Cost of kiln at W. S. George Pottery Co., Canonsburg, Pa., in 1951 was $60,000. - Estimated cost of mechanization would be $150,000, charged off equally over a 10-year period.
- Handling of raw materials and pelletization would be on a 1-shift, 5-day-week basis.
- The shift supervisor would also handle operation of the kiln.
- Seventy-five percent of the nickel oxide would be pelletized, using 8 pounds of starch and 10 pounds of bentonite per ton.
- Average life of sagger would be 3 months; milkstools, 1 year; and hillers, 4 months.
- Charred coal would not be reused.
The five grades of sponge nickel, weighing 316,235 pounds and containing 292,635 pounds of nickel plus cobalt, were melted in a 4-ton, tilting, basic-open-hearth furnace to produce nickel ingots. The open-hearth furnace was used to take advantage of its oxidizing characteristic for removing cobalt from the metal by selective oxidation.
On the basis of the results from the melting operation, especially removal of cobalt by other than slag preference, it is recommended and assumed for cost purposes that an electric-arc furnace would be used for melting.
Table 13 gives the costs of melting sponge nickel in the 4-ton open-hearth furnace with the furnace loaded to 40 percent of capacity. It required 36 operating days to complete the melting, with full melting crews on a 2-shift basis and furnace heater and helper on the third shift.
The operating crew consisted of a melter, 2 helpers, and 3 laborers, and the heating crew consisted of a helper and a laborer.
The costs given for the Bureau of Mines melting operations do not include capital costs, any charge toward the refractory that was in the furnace, or loss of nickel in melting.
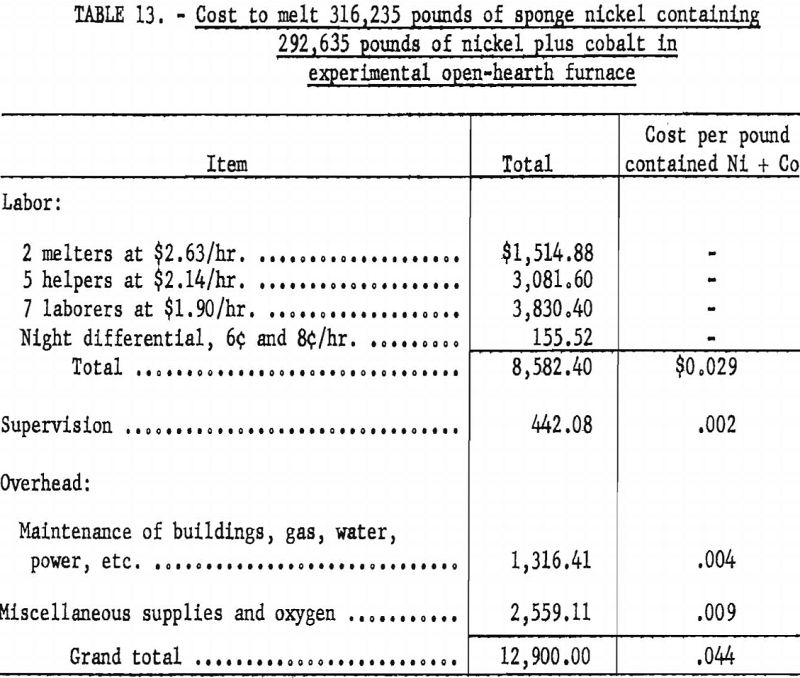
Table 14 gives the estimated cost of melting sponge nickel in an electric-arc furnace.
It can be assumed that melting sponge nickel would be easier than melting sponge iron; therefore, costs obtained for melting sponge iron, which were used as a basis for estimating, would be equal or higher.
Main operating difficulties in melting sponge iron Include the presence of gangue and iron oxide. This would not be a problem with sponge nickel, as the reduction is nearly complete, and gangue material is under 2 percent.
Melting temperature would be close to the melting point of pure nickel, as the carbon content would be under 0.10 percent.
The productive capacity (tons per hour) of a given furnace melting sponge iron was estimated at possibly 15 percent lower than when scrap is melted.
An electric furnace with an acid-steel rating of 3 tons per hour and a heat size between 9,000 to 12,000 pounds would be adequate.
