The reactions that take place during the dissolution of gold in cyanide solutions under normal conditions have been fairly definitely established. Most agree that the overall cyanide equation for leaching and cyanidation of gold is as follows:
4 Au + 8 NaCN + O2 + 2 H20 = 4 NaAu(CN)2 + 4 NaOH

In a relatively simple system of this type the gold dissolves readily. The only requirements are that the gold be free and clean, that the cyanide solution contain no impurities that might inhibit the reaction, and that an adequate supply of oxygen be present in the solution throughout the reaction period. Many gold ores in practice behave according to this reaction and the problems involved in the extraction of the gold are more mechanical than chemical. Many others, however, present an assortment of chemical problems depending on the various constituents in the ores. Many of these constituents such as quartz, silicate minerals, alkali metal carbonates are relatively inert to cyanide solutions. Other constituents, however, may react to a greater or lesser degree with the cyanide solution. Such constituents are frequently present in ores in amounts several thousand times greater than the gold present. This is particularly true at the present time because the trend is toward treating increasingly complex ores by cyanidation. In addition, the application of flotation and other methods of concentration to gold ores followed by cyanidation of the product thus obtained not only concentrate the gold but also frequently concentrate the detrimental impurities. The net result is the complication of the chemistry involved in the cyanide process.
Compare Cyanide VS Thiosulphate gold Leaching
No two ores are exactly alike. Therefore, it is practically impossible to predict how an ore will act during cyanidation, although broad generalizations can sometimes be made. In general, therefore, every ore presents its own problems in cyanidation.
 One of the most frequent sources of trouble, in cyanidation is the presence of copper minerals in an ore. The copper content may be less than 0.1011 hut its effect on both the dissolution and precipitation of gold can be very pronounced. Not only do copper minerals dissolve in cyanide and cause excessive consumption of this chemical, but also, the copper cyanogen complexes thus formed indirectly affect the dissolution of the gold. In addition, the copper in solution influences the precipitation of gold by zinc. The resulting gold precipitate contains copper which in turn frequently presents a problem in the subsequent melting operation.
One of the most frequent sources of trouble, in cyanidation is the presence of copper minerals in an ore. The copper content may be less than 0.1011 hut its effect on both the dissolution and precipitation of gold can be very pronounced. Not only do copper minerals dissolve in cyanide and cause excessive consumption of this chemical, but also, the copper cyanogen complexes thus formed indirectly affect the dissolution of the gold. In addition, the copper in solution influences the precipitation of gold by zinc. The resulting gold precipitate contains copper which in turn frequently presents a problem in the subsequent melting operation.
Zinc also forms cyanogen complexes but their effect on the dissolution of gold is much less marked than those of copper. Zinc complexes are more likely to cause trouble in the cyanidation of silver ores because such ores contain much more silver than a gold ore contains gold. Thus, a much larger amount of zinc is required to precipitate the dissolved silver, resulting in cyanide solutions carrying a relatively high percentage of zinc cyanogen complexes and in some cases zincates.
It has been suspected in practice that a small amount of nickel on a pregnant solution has a very detrimental effect on the precipitation of gold but it apparently has little effect on the dissolution of gold. The reason for this is not definitely known, nor is the cure.
The detrimental effect of nickel on the precipitation of gold can be controlled, however, by keeping the nickel content of the pregnant solution below a certain danger percentage. This is done by discarding barren solution at regular intervals. Although this procedure circumvents the problem, provided that the amount of nickel dissolved in one cyanidation cycle is not in excess of the critical amount, it does not solve the problem.
Arsenic in the form of realgar and orpiment and antimony as stibnite present serious problems in cyanidation. They do not form complex cyanides as far as is known but they do dissolve in alkaline solutions of the strength used in cyanidation to form compounds such as thioarsenites and thioantimonites. These compounds react with the oxygen in the cyanide solution to form the corresponding arsenites and antimonites the result is that practically no oxygen is available for the dissolution of gold, the extraction of which falls off accordingly.
Many ores contain carbonaceous material, sometimes in amounts varying from a few hundredths of a percent to several percent. In some forms this carbonaceous material has no effect on cyanidation; in other forms it is an active precipitant for gold in cyanide solutions. It is always difficult in practice to determine whether all of the gold in a cyanidation residue can be attributed to a refractory form of gold in the ore or whether some of it was first dissolved by the cyanide solution and then precipitated by carbonaceous material present. If the latter were the case, it would be possible to prevent this gold loss, at least to some extent.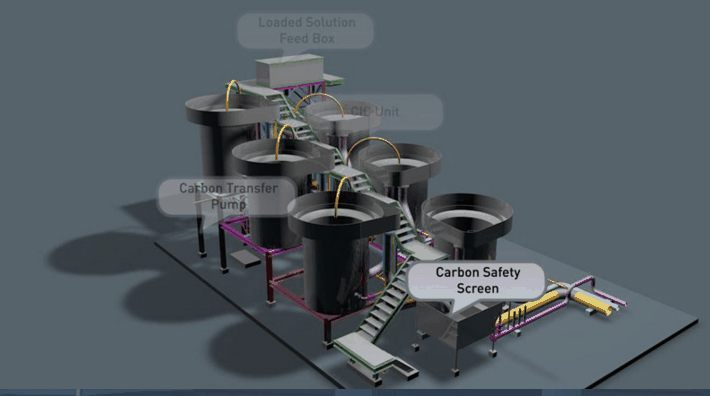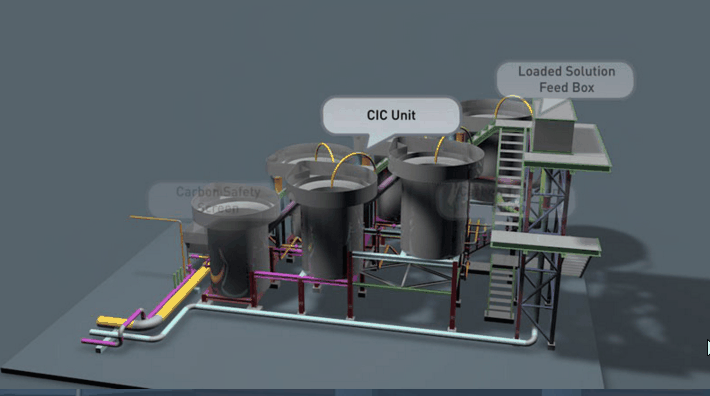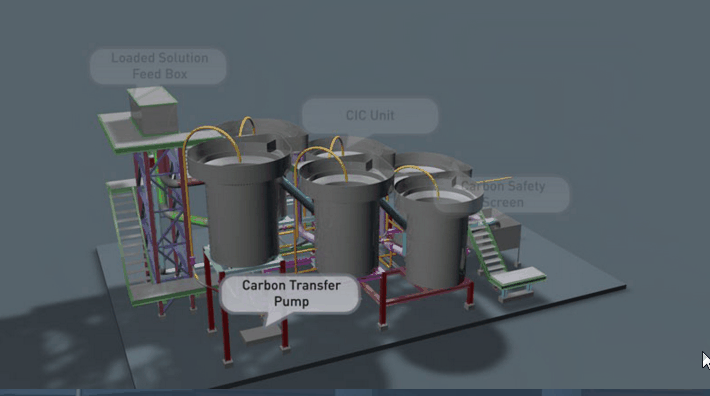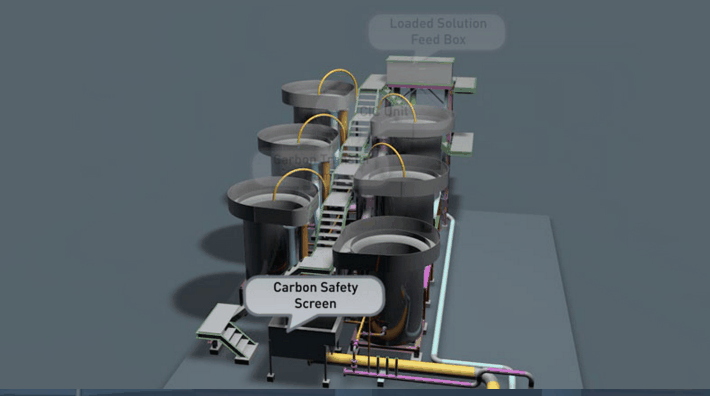
When sulphide minerals such as covellite, pyrrhotite, sphalerite, etc. are decomposed by, or dissolved in cyanide solutions, some of the products of the reactions, in addition to cyanide complexes, are alkaline sulphides, thiosulphates, thiocyanates. Some of these affect adversely’ the dissolution and precipitation of gold. Various means have to be used to accelerate their decomposition into relatively innocuous compounds.
Products obtained from gold ores by flotation contain, in addition to gold and sulphide minerals, a large proportion of the reagents used for the flotation process. Such reagents are xanthates, dithiophosphates, frothing oils, copper sulphate and others. Reagents such as xan- thate which have rendered the gold floatable by making it water repellent have a similar effect in cyanidation. That is, they prevent to some extent contact between the gold particles and the cyanide solution thus retarding dissolution of the gold. Copper sulphate behaves differently; under flotation conditions much of it is found in the flotation concentrate, possibly as the hydroxide. In this form it reacts readily with cyanide thus increasing cyanide consumption.
Gold occurs in ores in many different forms and the scheme of treatment has to be worked out accordingly. Fine, free and clean gold presents no problem provided that the cyanide solution contains no detrimental impurities. Coarse gold is usually removed ahead of cyanidation by gold traps, blankets or other standard methods and is treated separately. Otherwise, the dissolution process would be prolonged unduly due to the concentration of coarse gold in the grinding circuit, and the danger of high gold cyanidation residues would always be present.
Quite often the gold in an ore is tarnished, or as it is termed “rusty”. This brownish tarnish or coating very often consists of oxides of iron, but black coated gold is not unusual. The effect of such coverings on the gold particles is to retard dissolution and usually to increase the gold content of the cyanidation residue. This gold is not only refractory to cyanidation, it is also difficult to amalgamate, and difficult to float by the usual reagents used in flotation.
Occasionally, panning of a cyanidation residue and examination by microscope shows bright metallic particles somewhat lighter in color than clean gold. These particles are usually described as electrum which is a gold-silver alloy containing 15 to 35% silver. Inasmuch as gold-silver alloys of this composition are almost as soluble in cyanide as gold is, it is probable that some other factor has affected the solubility of the electrum particles. Possibly some other alloying element has influenced their solubility in cyanide.
The only compounds of gold found in nature are the gold-silver tellurides. There are many of these, some of which contain small amounts of other elements such as antimony, lead and mercury. Gold in the form of telluride is usually much more difficult to dissolve than metallic gold. Special procedures and processes have been used for the extraction of gold from ores containing tellurides.
Metallic gold is frequently associated very intimately with such minerals as pyrite and arsenopyrite. In order to insure contact between the gold and cyanide solution, the ore must be ground very finely. It may not be feasible to grind the entire ore to the necessary degree of fineness. In such cases the ore may be ground sufficiently fine to liberate the gold-bearing mineral which is then floated or concentrated by some other means. This product can then be ground separately to the desired fineness and cyanided.
Sometimes even fine grinding will not liberate the gold to the desired extent. The usual alternative in this event is roasting the ore, or the gold-bearing concentrate. During a roasting operation the form of the mineral constituents is altered completely. New problems in cyanidation are presented. For example, the mineral pyrite which is relatively inert in cyanidation may alter partially to ferrous or ferric sulphate. In the altered form it is much more harmful in cyanidation. Again, chalcopyrite, the least objectionable of copper minerals in cyanidation, may alter in roasting to the oxide or sulphate of copper. If such copper is present in any appreciable amount, steps may have to be taken to remove it prior to cyanidation.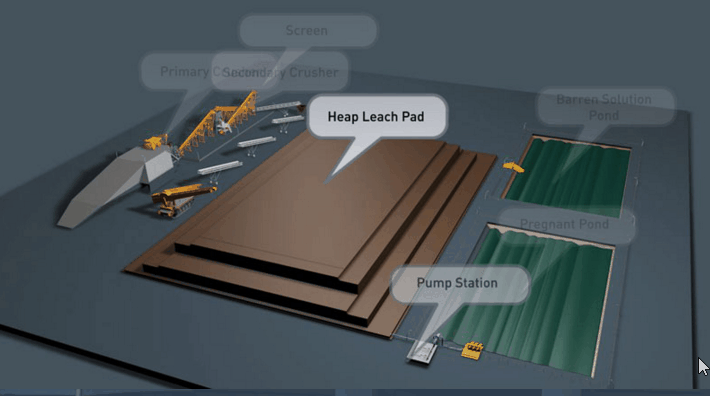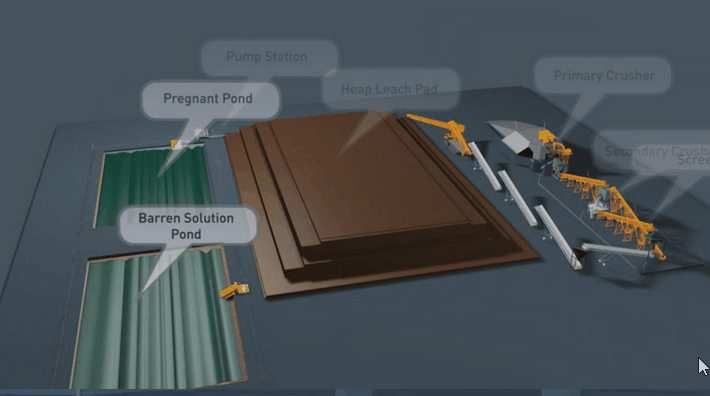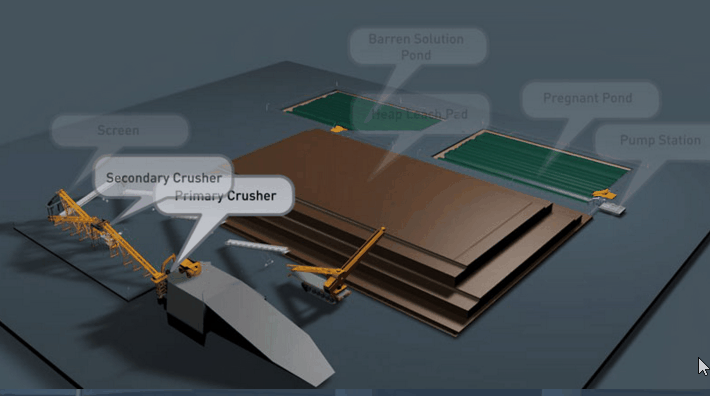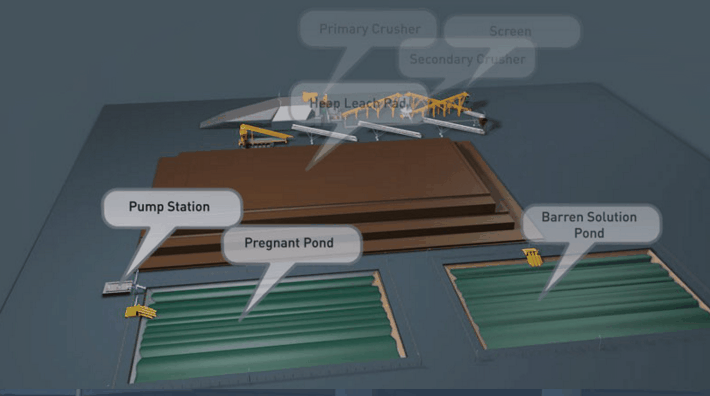
The reaction for the dissolution of metallic silver in cyanide solutions is analogous to that for the dissolution of gold. The occurrence of metallic silver in ores, however, is not common; the silver is more likely to occur in the sulphide form either as the plain sulphide or in combination with sulphides of copper, arsenic, antimony and lead. The reactions involved for the dissolution of such minerals are somewhat different from the reaction which takes place when metallic silver is dissolved in cyanide and conditions have to he regulated accordingly.
One of the most refractory forms of silver occurs as an oxide intimately associated with oxides of manganese. Cyanide solutions have practically no dissolving effect on silver of this type. Special leaching or roasting techniques have to be employed to liberate the silver before it will dissolve in cyanide solutions.
The water used in cyanidation plays an important part in the process. It may contain bi-carbonates, magnesium sulphate, organic matter, heavy metal salts, chlorides and other compounds, anyone of which might affect a cyanide solution to a greater or less degree, unless the necessary corrective steps are taken.
The analysis of ores for specific minerals is frequently useful to cyanide operators for the purpose of tracking down variations in mill operation. For example, an ore might contain both pyrite and pyrrhotite. A difference in the total iron might be of no significance whatsoever, whereas a difference in the pyrrhotite content of the ore might affect seriously the operation of a mill. The determination of oxide copper in the presence of chalcopyrite, or oxide lead in the presence of galena, or oxide zinc in the presence of sphalerite might be equally significant.
The accurate analysis of a mill solution containing complex cyanides, for such compounds as soluble sulphides, thiosulphates, ferrocyanide, oxygen, presents one of the most difficult problems in cyanidation. Even the free cyanide content of such solutions is difficult to assess accurately. A knowledge of the various factors affecting these analyses is of primary importance to the operator.
The various problems that have been touched on briefly in this introduction will be discussed in detail in subsequent sections.
https://www.academia.edu/292086/Kinetics_and_Mechanism_of_Gold_and_Silver_Dissolution_In_Cyanide_Solution
https://open.library.ubc.ca/cIRcle/collections/ubctheses/831/items/1.0106728
