Table of Contents
Standard methods of analysis for calcium and magnesium are involved and time consuming and generally require considerable analytical skill. Investigation of possible alternatives more suitable for rapid routine application led to a procedure developed for the rapid determination of calcium, magnesium, and iron in limestone, in which these cations are determined by a series of simple titrations, and from which the proposed method was adapted.
The method has proved dependable and is considered to be simpler, faster, and more reliable than the application of flame photometry to this problem. Moreover, only usual analytical equipment is needed the proposed method does not require expensive or complicated instruments or other than basic analytical skill.
With the proposed method, calcium and magnesium are determined by titrations based on the complexing action of EDTA ((ethylenedinitrilo) tetraacetic acid disodium salt) at appropriate pH levels. A preliminary analytical separation (treatment with ammonium hydroxide, ammonium persulfate, and ammonium sulfate) isolates the calcium and magnesium from the usual co-constituents iron, aluminum, manganese, titanium, and phosphorus, all of which could interfere with the determination. Chromium, vanadium, or molybdenum, when present, are removed by the initial treatment of the sample (procedure A); and their interference with the calcium, magnesium, or titanium determinations so prevented. Interference due to nickel, cobalt, or copper can be obviated by the addition of potassium cyanide before the titrations for calcium and magnesium are begun.
The intentional introduction of the sulfate ion (in the above-mentioned preliminary separation) removes virtually all barium and almost all strontium as relatively insoluble sulfates. Loss of calcium having sulfate of limited solubility is prevented by maintaining the solution volume high enough (300 ml.) to insure solubility of calcium sulfate. The slight amount of strontium remaining in solution would titrate with the calcium, causing this value to be somewhat high. This positive error would be small and nearly always could be ignored, since strontium usually is not present in significant amount in the materials for which the method was designed.
In a volume of about 300 ml. of water the limit of solubility of calcium sulfate is about 0.63 gram, or the equivalent of 0.26 gram as calcium oxide 52 percent on a 0.5000-gram sample basis; consequently, the sample weight taken should be such that this concentration is not exceeded greatly. For higher calcium oxide concentrations than this value, a smaller sample weight should be used.
Except for portland cement, the other material compositions usually permit a standard sample weight of 0.5000 gram. For material compositions very low in both calcium and magnesium oxides, a larger weight of sample may be taken. In this instance, however, the greater concentration of other constituents, particularly iron, may require that additional or special analytical treatment be made.
Preliminary Treatment of Sample
Procedure A
General application for determination of calcium, magnesium, and titanium: Material compositions high in aluminum or phosphorus or containing more than slight amounts of chromium, vanadium, and/or molybdenum should be treated by this procedure, rather than by the shorter procedure B described later, even if the determination for titanium is not required.
Step 1-A
Weigh 0.5000 gram of the properly prepared sample (at least 60-mesh) into a 25-ml.-capacity platinum crucible containing about 10 grams of sodium carbonate if an oxidizing fusion is indicated, as for materials containing appreciable chromium, add no more than 0.5 gram of sodium peroxide and mix thoroughly. Cover crucible and fuse for at least 10 minutes after initial melting; some materials of complex composition and high-alumina slags may require a longer fusion time. Materials containing appreciable carbonaceous matter should be weighed into an empty crucible, and the organic matter destroyed by ignition before mixing with the flux.
Step 2-A
Remove crucible cover and then, as rapidly as possible, pour the still-molten charge into a small (50 to 100 ml.) platinum dish resting in a tray of cold water, covering as large an area as possible. Immediately cover the dish to avoid loss by splatter that may occur with rapid cooling. Transfer without loss the cooled melt, crucible, and cover to a 400-ml. beaker about half filled with hot water.
Alternatively, the molten charge may be granulated by pouring it directly into a 400-ml. beaker about half filled with cold water and immediately covering the beaker. Since this operation could be hazardous, especially when pouring oxidizing fusions, a face shield or other adequate protection must be provided. With proper technique, however, the danger of an explosion and consequent loss of sample is remote, and the reduction in time required to leach the melt is appreciable.
Regardless of the method used, heat beaker to incipient boiling—the insertion of a stirring rod with a roughened or etched tip will minimize bumping. After disintegration of the melt, cool somewhat and remove crucible and cover, dislodging any adherent residue by scrubbing with a policeman-tipped rod and rinse back into beaker. Reserve crucible. Add to the beaker about 2 grams of sodium carbonate and 2 grams of sodium hydroxide pellets, stir for 1 to 2 minutes, and then filter through a moderately retentive paper, such as Whatman No. 40 or equivalent. The sodium hydroxide is added before filtering so that the relatively insoluble magnesium hydroxide is formed. Wash paper and residue about four times with warm water containing about 2 grams of sodium hydroxide and 2 grams of sodium carbonate per 100 ml.
The filtrate, which is discarded, contains any vanadium and molybdenum, and, if an oxidizing fusion is employed, also chromium; also most of the phosphorus, most of the aluminum, an indefinite portion of the silicon, and the excess sodium salts. The residue retains the calcium and magnesium (as the insoluble carbonate and hydroxide), along with the iron and titanium and generally most, if not all, of the manganese. In addition, the residue will contain those portions of the silicon and aluminum not removed by the basic aqueous extraction, and perhaps a trace of the phosphate ion. Separation of the calcium and magnesium from these constituents is accomplished in step 3-A; separation from the silicon is incomplete, but this has no adverse effect.
Transfer the paper and residue back into the original beaker; macerate paper in about 40 ml. of 1:1 hydrochloric acid added in portions from the reserved platinum crucible so that any adherent film or stain is removed therefrom. Heat beaker gently for several minutes until the residue has dissolved. Some silica may separate at this point, but this is of no consequence. Dilute with hot water to a volume of at least 300 ml. Since the sulfate ion is introduced in the next operation, this minimum volume must be maintained to insure solubility of calcium sulfate.
Step 3-A
Neutralize with ammonium hydroxide and add about 8 ml. excess. Enough ammonium chloride is formed during neutralization to prevent coprecipitation of the calcium and magnesium. Bring solution to a boil and add about 20 ml. of a freshly prepared 25-percent ammonium persulfate solution and about 0.5 gram of ammonium sulfate. Boil 1 to 2 minutes after this addition (the solution should still be strongly ammonical), and filter through a rapid paper (Whatman No. 41-H), retaining filtrate in a 600-ml. or larger beaker.
For the routine analysis of most blast-furnace slag compositions and other materials yielding a relatively low volume of precipitate in this step, a single precipitation is generally sufficient. However, a second precipitation should be made, particularly with voluminous precipitates, unless previous investigation of the precipitate showed retention of calcium and magnesium to be negligible for any given material composition.
Wash paper and precipitate about 10 times with a hot, very slightly ammonical, 2-percent ammonium chloride solution. If analysis for titanium is to be made, transfer paper and precipitate to the original 400-ml, beaker and proceed as directed under Determination of Titania; otherwise discard. Boil the filtrate as vigorously as possible—a stirring rod with a roughened or etched tip and a ribbed or raised cover glass aids materially in minimizing bumping for at least 30 minutes. This boiling period is required to decompose the excess persulfate ion and to reduce sufficiently the solution volume, if necessary, to permit transfer to a 500-ml. volumetric flask. After such a transfer of the cooled solution has been made, adjust to volume at about 20° C. Aliquots of this solution are taken for the lime and magnesia titrations, as later described.
Procedure B
Specific application for determination of calcium and magnesium in materials containing little or no chromium, vanadium, or molybdenum, and not unusually high in aluminum or phosphorus. Most blast-furnace slag compositions are amenable to this procedure, which is considerably faster than the general one.
Step 1-B
Weigh a 0.5000-gram sample into a 400-ml. beaker and add about 40 ml. of water and about 2 grams of sodium chloride. Bring to a rapid boil and add 20 ml. of concentrated hydrochloric acid. Continue heating, continuously swirling beaker to avoid clumping or agglutination, until the sample has been decomposed. Solution of sample, except for possible separation of gelatinous silica, should be complete in several minutes.
If the sample (such as the mixed standard used to obtain the calcium oxide and magnesium oxide titers of the solution used in the subsequent titrations) is not completely decomposable by this acid treatment, the same weight of sample should be treated as follows: Fuse in a 15-ml.-capacity platinum crucible with as little sodium carbonate as possible (about 4 grams) for about 10 minutes or longer if necessary. Leach crucible and melt in about 60 ml. of 1:1 hydrochloric acid. Dilute solution obtained by either process to about 300 ml. with hot water and proceed as directed in step 3-A, procedure A.
Determination of Lime (CaO)
Reagents Required
- EDTA titrating solution: Dissolve (using 4 grams of the disodium salt of (ethylenedinitrilo) tetraacetic acid per liter of water) enough reagent to give the desired volume of titrant. Standardize this solution against a material of known composition as described later under standardization. The solution is stable, and ordinarily only initial standardization is required.
- Potassium hydroxide: 20-percent aqueous solution; store in plastic bottle.
- Potassium cyanide: 10-percent aqueous solution; store in a dropping bottle.
- Modified calcium-indicator powder: Mix thoroughly 0.2 gram of calcein (now available commercially through scientific supply outlets) with 0.12 gram of thymol pthalein and 20.0 grams of potassium chloride.
Titration Procedure
Pipet a 25-ml. aliquot from the 500-ml. volumetric flask (step 3A) into a 250-ml. beaker. Add, in order, about 10 ml, of the potassium hydroxide solution (the pH should be maintained above 12), several drops of the potassium cyanide solution, and a tiny amount (10 to 20 mg.) of the modified calcium-indicator powder. The color at this point should be light purple with a definite greenish fluorescent cast. Stir and titrate with the EDTA solution to a clear purple and the complete disappearance of any greenish fluorescence.
The titration to the end point is best followed by looking directly into the solution from the top rather than obliquely through the beaker sidewalls. A titration light source providing indirect lighting (fluorescent) proved to be satisfactory for both calcium and magnesium end point observation. Observation of the calcium end point was improved by setting the beaker on a small piece of black rubber matting covering the white base of the titrating stand. The volume of EDTA required for titration, multiplied by the calcium oxide titer obtained in the standardization (as described later) gives the calcium oxide directly in percent, based on a 0.5000-gram sample.
Determination Of Magnesia (MgO)
Reagents Required
- EDTA titrating solution: As described under the determination of lime.
- Potassium cyanide: As described under the determination of lime.
- Buffer solution: Dissolve 60 grams of ammonium chloride in 200 ml. of water, add 570 ml. of concentrated ammonium hydroxide and dilute to 1 liter with water. Store in a plastic container.
- Titrating indicator solution: Dissolve 0.15 gram of Eriochrome Black T (available commercially same sources as for Calcein) and 0.5 gram of sodium borate in 25 ml. of methanol. Because of its relative instability, this solution should not be kept longer than 3 or 4 days.
Titration Procedure
Pipet a 25-ml. aliquot from the 500-ml. volumetric flask (step 3-A) into a 250-ml. beaker. Add, in order, 5 to 6 ml. of buffer solution, several drops of potassium cyanide, and 6 to 10 drops of the titrating indicator solution. Stir and titrate with the EDTA solution. The end point is reached when the color changes from wine-red to pure blue. A white matte surface, such as a sheet of filter paper, should be interposed between the bottom of the beaker and the base of the titration stand. Otherwise, the same end point observation and titrating technique used for the lime titration also applies here.
Actually, the volume of EDTA required for this titration is for both lime and magnesia. Therefore, subtract from this value the number of milliliters required in the lime titration to obtain the volume required for magnesia alone. This volume, multiplied by the magnesium oxide titer obtained in the standardization (later described), gives the magnesium oxide directly in percent, based on a 0.5000-gram sample.
Standardization of Edta Titrating Solution
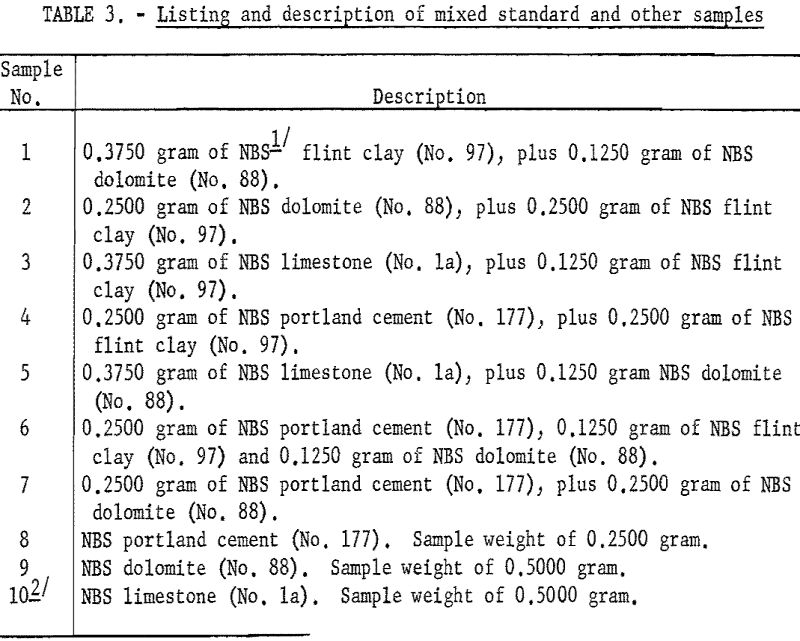
Weigh 0.2500 gram of NBS (National Bureau of Standards) standard sample la (limestone) and 0.2500 gram of NBS standard sample 88 (dolomite) and combine as one 0.5000-gram sample. Carry this mixed standard through all steps of the particular procedure (A or B) used. The factors or titers obtained by procedures A or B may differ slightly, and for this reason the EDTA titrating solution should have the factors established for the same procedure(s) employed in the actual analysis.
To obtain the calcium oxide factor directly in terms of percent, divide the percentage of calcium oxide present in the mixed standard (35.90) by the volume of EDTA required in the determination of lime. To obtain the magnesium oxide factor directly in terms of percent, divide the percentage of magnesium oxide present in the mixed standard (11.84) by volume difference between the total volume required in the determination of magnesia and the volume required in the determination of lime. The order of magnitude of the calcium oxide factor is generally 2.4-percent CaO per ml.; that of the magnesium oxide factor, 1.6—percent MgO per ml. Instead of the mixed standard, another material of known composition, preferably similar in constituent type and range to the compositions under analysis, may be used.
Determination of Titania (TiO2)
Macerate the filter paper and precipitate contained in the reserved 400-ml. beaker (step 3-A, procedure A) with about 10 ml. of concentrated hydrochloric acid and 10 ml. of concentrated nitric acid. Heat moderately several minutes or until pulped paper has begun to disintegrate. Add about 30 ml. of perchloric acid (72 percent) and continue moderate heating until paper has disappeared. Heat strongly until fumes of perchloric acid have been evolved for about 10 minutes. A wet oxidation of organic matter, such as filter paper, proved to be a safe, efficient, and rapid process if carried out in this manner. Cool beaker somewhat and dilute to about 100 ml. with hot water. Add about 1 ml. of sulfurous acid and then boil for several minutes to remove excess sulfur dioxide. Filter off the residue (essentially silica) through a rapid filter paper, such as Whatman No. 41 H or equivalent, and wash about 10 times with hot water. Retain filtrate in a 250-ml. volumetric flask. Discard the residue. Adjust volume to 250 ml. at about 20° C, Take two 25-ml, aliquots, transfer one to a 50-ml, volumetric flask marked A and the other to a similar flask marked B. Add 5 ml. of 3-percent hydrogen peroxide to B and then adjust both flasks to volume with water. Mix well.
Use solution A to obtain the blank setting on a suitable spectrophotometer or colorimeter (a Beckman Model B spectrophotometer was used in this work) at a wave length of 410 mµ. Use solution B to obtain the absorbance reading. From a prepared calibration curve or by use of an appropriate factor, obtain the value for titanium oxide corresponding to this absorbance. The factor used was obtained from a plot of concentration versus absorbance values, which was essentially a straight-line relationship throughout the range studied. The calibration is accomplished by reading the absorbance of solutions, which varies enough in TiO2 content that a curve of the desired range can be established. These solutions, which should contain the elements that normally would accompany titanium, such as iron and manganese, in concentration similar to the material under analysis, are best prepared synthetically. Variation in titania content is accomplished by adding increments of a standard titanium solution of suitable concentration to these solutions.
Discussion of Results
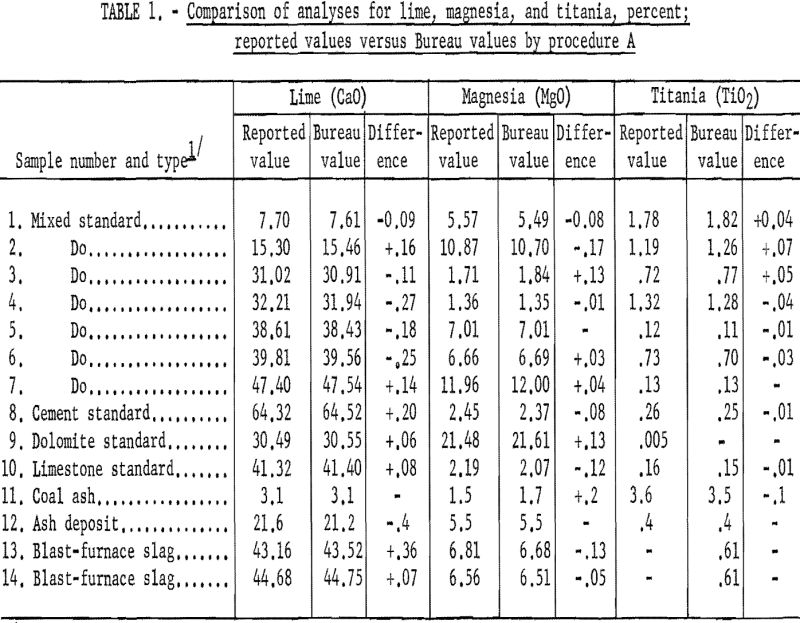
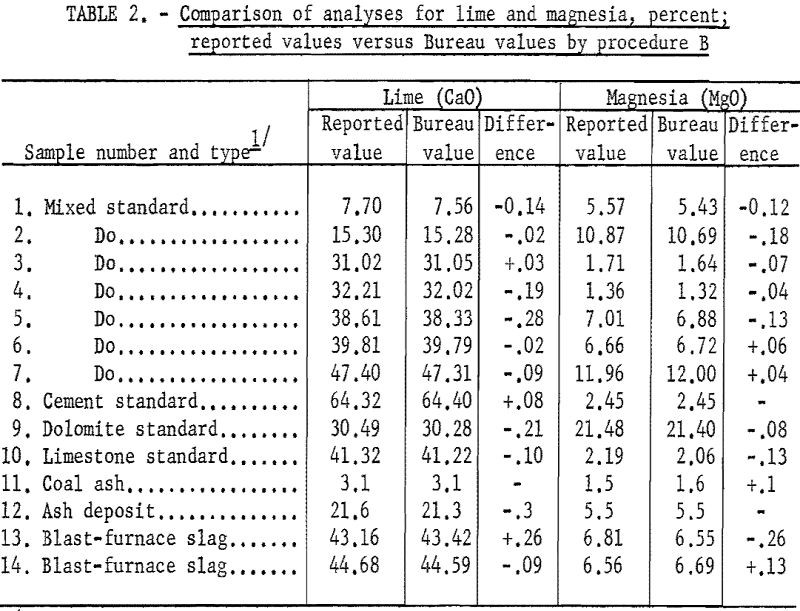
Table 1 (procedure A) and table 2 (procedure B) show that the results obtained by the Bureau method are generally in good agreement with the reported values obtained by conventional methods. The difficulty experienced in obtaining accurately analyzed slag or other material compositions with sufficient range in lime and magnesia (and titania) led to the use of standard analyzed samples to supply most of the reported values.
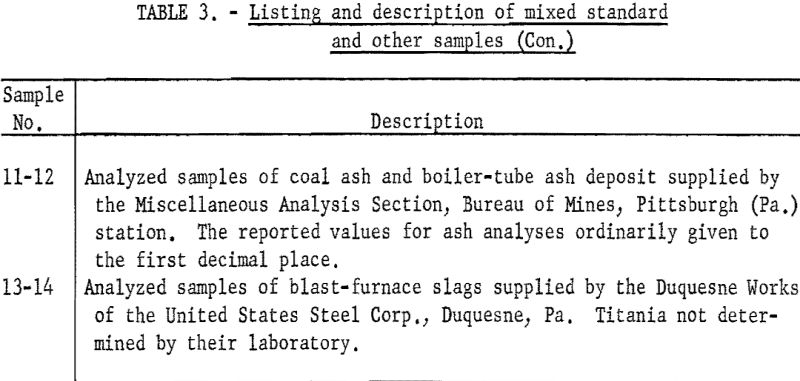
As shown in table 3 the samples numbered 1 through 7 were prepared from standard samples (NBS) by combining appropriate weights of suitable individual standard samples to give a range of accurately established values. All the results listed as Bureau values were obtained by the proposed method and are the average of four determinations for each constituent per sample for each procedure. The individual agreement of the four determinations was within plus- or minus-0.5 percent of the amount present for the highest values given and within plus- or minus-1.0 percent of the amount present for the lowest values. The results listed as reported values are averages; the number of individual determinations and their agreement was available only for the NBS standard samples and can be found in the certification of analysis furnished with each standard sample.
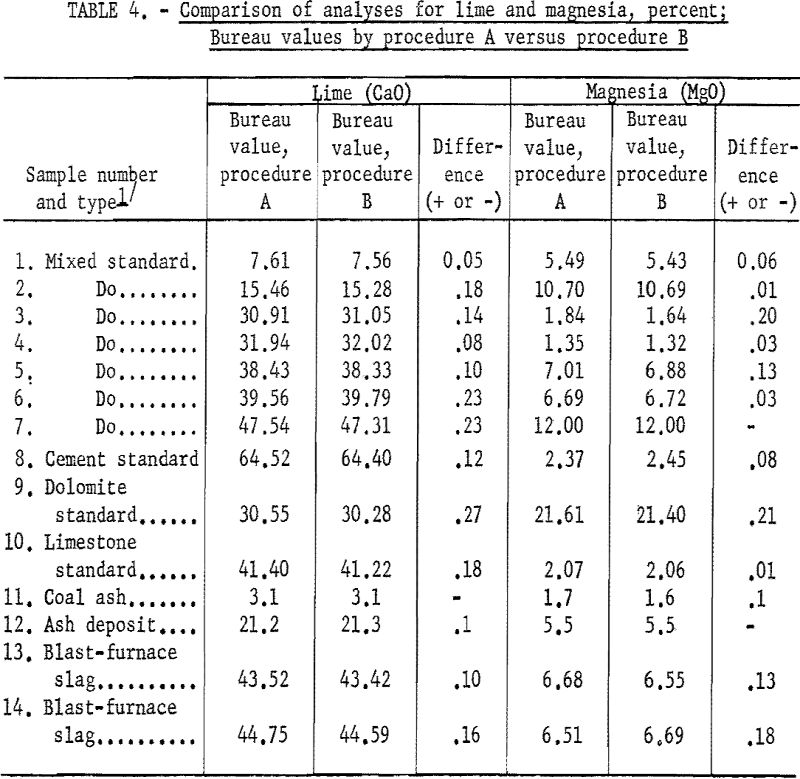
Table 4 shows the agreement of Bureau values for lime and magnesia between procedures A and B.
Conclusions
The proposed method allows analyses for lime and magnesia to be made in much less time than is possible with standard methods and with comparable accuracy. Standard methods seldom permit these analyses to be made in less than 6 hours and then only if some of the analytical procedure can be shortened or omitted. If all precautions of precise or umpire analysis were followed, as with standard samples, several days would be required. The time required by the proposed method for the generally applicable procedure (A) is about 3 hours—slightly longer if the optional determination for titania is included. The more specific procedure (B) usually requires less than 2 hours and is suitable for most blast-furnace slag compositions. Generally, the shorter the time interval in which these analyses can be made and reported the greater value they have in application to the operation of a blast furnace or to any other process in which such information is desired.
The method is especially adapted for routine use, since it is simple and reliable and the operator needs no special analytical qualifications; moreover, only usual analytical equipment is needed.
The proposed method is applicable also to other lime- and magnesia-bearing materials such as limestone and dolomite fluxes, coal and coke ash, blast-furnace dust, portland and high-alumina cements, and many open-hearth and electric-furnace slag compositions.