This research was conducted as a part of a Bureau of Mines aim to promote more efficient use of mineral-based materials. The objective of the study was to prepare high-strength, creep-resistant lead utilizing the coprecipitation method of dispersion strengthening. The excellent corrosion resistance of lead and its sound attenuation capability, easy formability, and other advantageous properties have not been fully utilized in many applications owing to its poor creep resistance and low strength. Improvement of these properties would permit the use of lead in areas where scarcer, more expensive, materials are now used and would broaden the areas of potential application of lead.
Dispersion strengthening has been most successful in maintaining the strength of metals at temperatures near their melting points. Examples of this are SAP and TD nickel. The need for maintaining strength near the melting point is more important for lead than for higher-melting-point metals. At ambient temperatures, lead is already at approximately half its melting point on the absolute temperature scale. Thus, dispersion strengthening offers an excellent means of improving strength and creep resistance of lead in the range of temperatures at which the metal is most often used.
Lead has been successfully dispersion-strengthened with lead oxide copper 3 and aluminum using powder metallurgy methods. Another method of producing dispersions in lead is the coprecipitation process described in this report.
Previous work by Bureau of Mines personnel and others has demonstrated that the coprecipitation process may be successfully utilized to dispersion-strengthen copper and other metals. In April 1968, a patent was granted to Alexander of the Du Pont Co. which describes the dispersion strengthening of low-melting-point metals (including lead) by the precipitation of oxygen-containing compounds upon filler particles contained in a colloidal aquasol. The resulting precipitates are hydrogen-reduced and processed by powder metallurgy techniques. The method of producing dispersions in lead described in the present report involves the simultaneous precipitation of lead carbonate and aluminum hydroxide followed by roasting to oxides and hydrogen reduction of the lead oxide. The resulting powder is processed by powder metallurgy methods.
Experimental Procedures
Starting solutions were made from reagent-grade chemicals and distilled water. Weights of Pb(NO3)2 and Al(NO3)3·9H2O calculated to yield 1 kg of Pb-Al2O3 powder were added to 6 liters of water for the starting solution. The precipitating solution consisted of 696 grams of (NH4)2CO3 and 1,080 cu cm of 5 M NH4OH in approximately 6 liters of distilled water. The amounts of (NH4)2CO3 and NH4OH were experimentally determined for complete precipitation of Pb(CO3) and aluminum hydroxide. Volumes of metal salt solution and precipitating solution were maintained approximately equal to facilitate mixing.
Figure 1 is a schematic drawing of the apparatus used for coprecipitation. This is identical to apparatus used in a previous Bureau of Mines investigation concerning dispersion-strengthened copper. The Pb(NO3)2-Al(NO3)3 solution was combined with the precipitating solution in a blender where precipitation occurred. The flow rates of the two solutions were controlled by stopcocks. The pH of the combined solutions was maintained between 7 and 8 by adjusting flow rates. The solution containing precipitate was siphoned out and filtered. Coprecipitation and siphoning were conducted simultaneously.
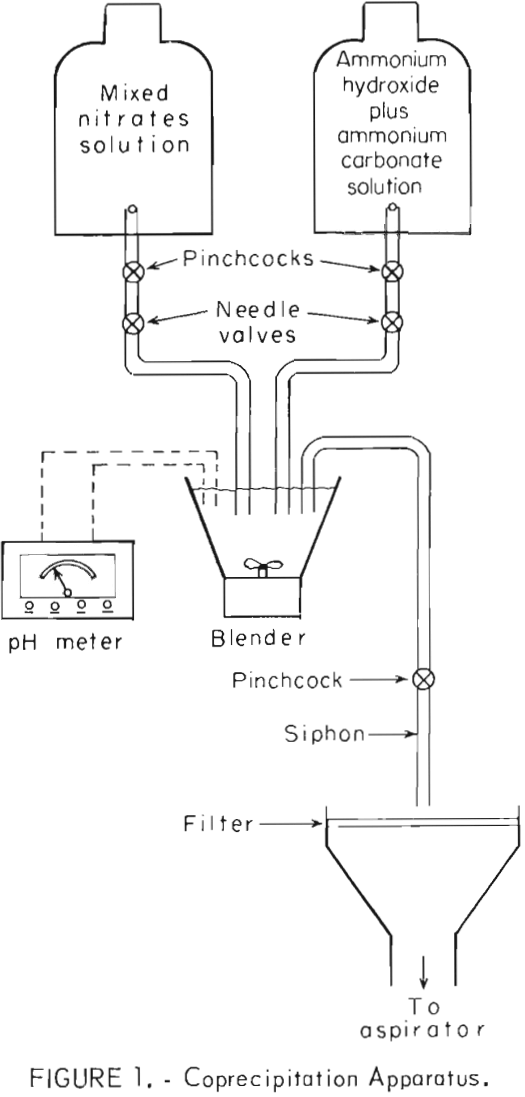
The precipitates were washed twice in distilled water, refiltered after each washing, and dried for about 15 hours at approximately 120° C. The precipitates were identified as PbCO3 by X-ray diffraction analysis. Volume-percentages of aluminum hydroxide were too small to be detected. The precipitates were calcined at 450° C for 1-½ hours and at 600° C for ½ to ¾ hour to drive off water and nitrogen oxides, and to convert PbCO3 and aluminum hydroxide to oxides. The calcined precipitates were identified by X-ray analysis as PbO. Again, volume-percentages of Al2O3 were too low to be detected by X-ray techniques.
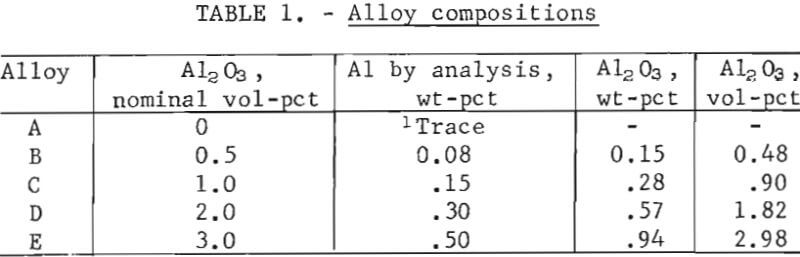
Reduction was carried out in hydrogen at 300° to 315° C for 18 to 24 hours. The reduced powders were allowed to cool in the furnace under a hydrogen atmosphere and immediately transferred to a vacuum desiccator for storage. Weight-percentages of aluminum in the hydrogen-reduced powders were determined by chemical analysis. The assumption was made that aluminum was present as Al2O3, and volume-percentages were calculated on this basis. Compositions are shown in table 1. One batch of lead powder was made without Al2O3 addition and processed in the same manner as Pb-Al2O3 alloys.
The reduced powders were iso-statically pressed at 60,000 psi into billets approximately 1-½ to 1-¾ inches in diameter by approximately 2½ inches long.
Preceding extrusion, the compacted billets were given a sinter-reduction in hydrogen at 300° to 315° C for approximately 24 hours They were allowed to furnace-cool in the hydrogen atmosphere and extruded at room temperature immediately upon removal from the furnace. Billets were extruded to ¼-inch- diameter rod at an extrusion ratio of 64 to 1 and an average pressure of 70 to 115 tons.
To provide test specimens, sections of the extruded rod were rolled at 125° C to 0.0625±0.0005-inch-thick strips for a total reduction of 75 percent. Creep and tensile specimens were cut from the rolled strip. Rolling was originally attempted at room temperature, but considerable edge cracking of the strip made it impossible to obtain good tensile specimens. Some edge cracking was also apparent in strip rolled at 125° C, but the defective areas were cut away during machining.
Creep testing was conducted at a constant temperature of 90°±3° F. Specimens were stressed up to 4,000 psi in creep tests. Tensile testing was conducted at a strain rate of 0.1 inch per inch per minute.
Metallographic samples were sectioned with a hacksaw, ground by hand on silicon carbide papers, and polished with diamond abrasives. The samples were
etched with a mixture of 10 percent hydrogen peroxide and acetic acid in a 1-to-1 ratio.
Results
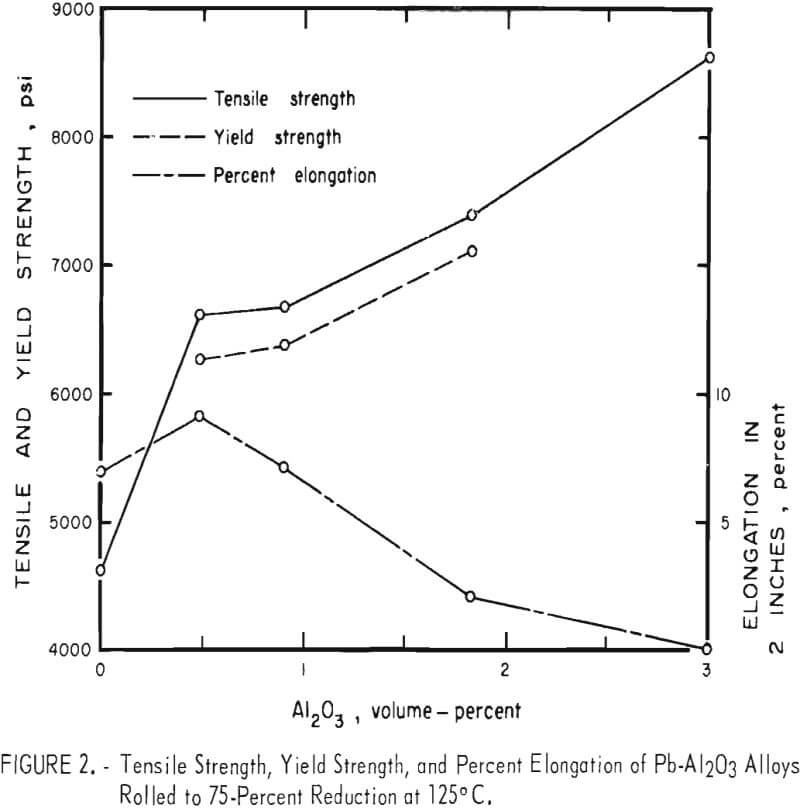
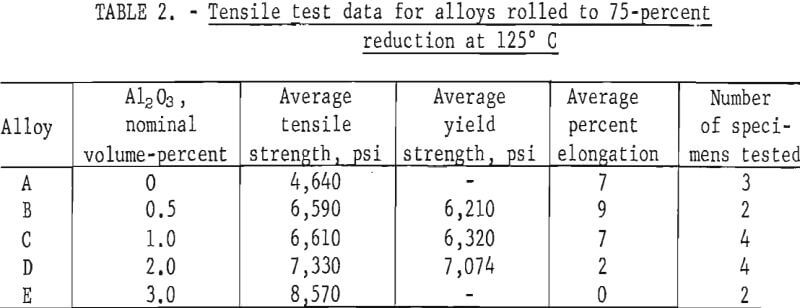
Tensile test results are listed in table 2 for Pb-Al2O3 alloys that were given a 75-percent reduction at 125° C. These results are plotted in figure 2. Alloy A made by precipitation of PbCO3, but without Al2O3 , had an average tensile strength of 4,640 psi versus 2,961 psi for chemical lead. Micro-structures of rolled alloy A show a fine-grained lamellar structure containing coarse, nonmetallic inclusions. Spectrographs analysis of this material reveals trace amounts of Al, Cr, Cu, Fe, Mg, and Si. Lead oxide or oxides of any of the trace elements could effectively dispersion-strengthen the unalloyed lead. Fine grain size is also undoubtedly contributing to the tensile strength of alloy A. Lead oxide could be present as a result of incomplete reduction, or the reduced lead powder could have been slightly oxidized during handling. The addition of 0.5 volume-percent Al2O3 to precipitated lead powder results in a 42-percent increase in tensile strength without loss of ductility compared to precipitated lead powder without Al2O3 addition. Additional amounts of Al2O3 up to 3 volume-percent result in greater strength increases but with a corresponding decrease in ductility. At 3 volume-percent Al2O3 there is no measurable elongation.
Yield strengths were not obtained for Pb without Al2O3 addition and Pb-3Al2O3. Edge cracks on specimens of these compositions made it necessary to position the tensile test grips close to the reduced sections of the specimens, which did not allow space for an extensometer.
Results of creep testing Pb-Al2O3 alloys are shown in table 3 together with data for some common lead alloys and an oxide dispersion-strengthened lead. Each creep rate listed for the Pb-Al2O3 alloys is the result of a single test. Direct comparisons of creep rates are difficult because of different stress levels. However, it can be seen that the dispersion-strengthened alloys are superior to the other alloys. The lowest creep rate found for common lead alloys is that of Pb-0.028 Ca which has a creep rate of 0.1 percent per year at 360 psi. By contrast, at 3,000 psi, a Pb-2Al2O3 alloy has a creep rate of only 0.011 percent in 10,000 hours (10,000 hours = 1.14 years).
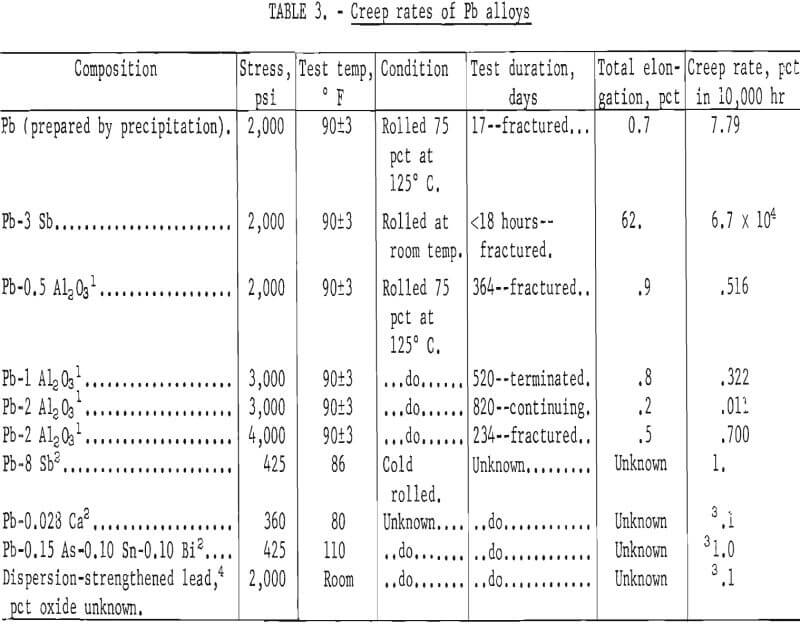
The time to fracture of 17 days at 2,000 psi and a creep rate of 7.79 percent in 10,000 hours, for the unalloyed lead prepared by precipitation, may be compared with a fracture time of less than 18 hours and a creep rate of 6.7 x 10 4 percent in 10,000 hours for a lead-3 percent antimony alloy. The superior creep resistance of the unalloyed lead may be taken as evidence of dispersion strengthening resulting from the method of processing.
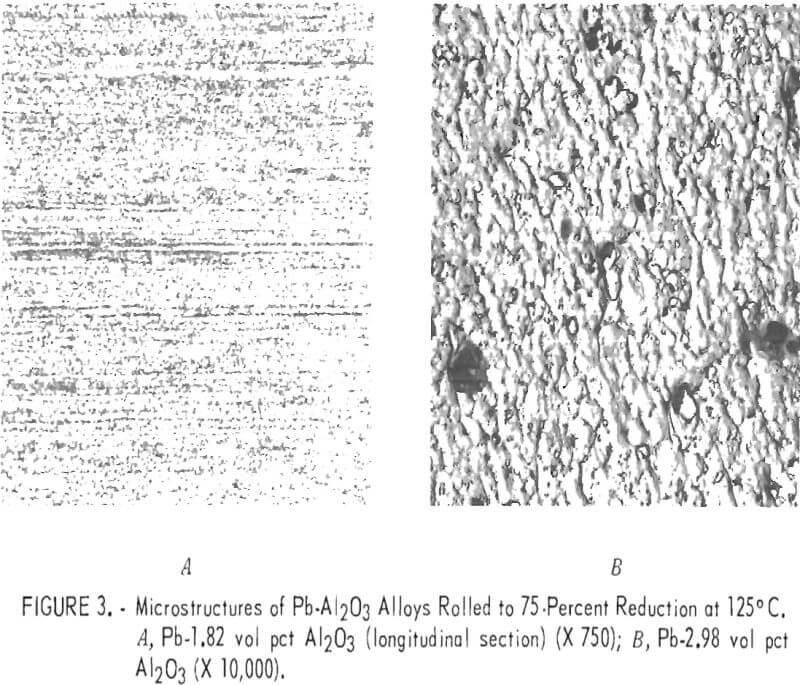
Figure 3A shows the microstructure of a rolled Pb-1.82 volume-percent Al2O3 alloy. The structure is indistinct but appears to consist of very small, irregularly shaped grains alined in the rolling direction. Al2O3 particles have probably restricted grain growth which would result from severe deformation of the lead matrix during extrusion and rolling. An electron micrograph is shown in figure 3B of a rolled Pb-2.98 volume-percent Al2O3 alloy. A few large particles are approximately ½ micron in diameter, but the majority appear to have diameters of about 1/10 micron or less. Since the material has been considerably strengthened, it can be assumed that slip and grain boundary motion have been restricted by the presence of this fine dispersion. A combination of small grain size plus restricted slip and grain boundary motion results in good creep resistance and strength but reduced ductility.
Conclusions
Lead produced by precipitation of PbCO3 from an aqueous solution of Pb(NO3)2, followed by roasting and hydrogen reduction, shows a considerable increase in tensile strength and a corresponding decrease in ductility compared to chemical lead. The coprecipitation of aluminum hydroxide with PbCO3 from an aqueous solution of lead and aluminum nitrates with subsequent roasting and hydrogen reduction results in additional increases in tensile strength. Creep resistance is greatly improved compared with that of conventional unalloyed lead and lead alloys. The coprecipitated material exhibits a decrease in ductility with increasing amounts of Al2O3. A good compromise between increased creep resistance and tensile strength versus reduced ductility can be obtained from dispersion-strengthened lead containing 0.5 to 1.0 volume-percent Al2O3.
