Table of Contents
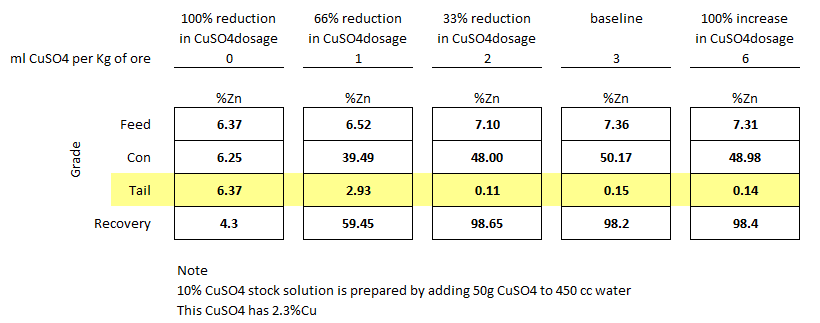
As an example of the beneficial effect of copper sulfate in the activation of zinc sphalerite toward flotation, the series of results shown in Fig. 1 is of interest. These represent rougher tests on a sample of heavy pyritic ore. The particular sample in question contained about 5.6% zinc as marmatite; and was treated in a circuit made alkaline with lime, using pine oil as a frother and, potassium amyl xanthate as a collector. As can be seen, in the blank test with no copper sulfate, less than 50% of the zinc was, contained in a rougher concentrate, analyzing only 8.5 % zinc. The addition of increasing amounts of copper sulfate increased both grade of rougher concentrate and extraction, until about 80% extraction was reached.

For higher percentages of extraction the grade of the rougher concentrate fell off. The proportions. of copper sulfate used are expressed on the face of the diagram in pounds CuS04.5H20/ton of ore. It will be noticed that most of the beneficial effect is obtained by as little as 1 lb. crystalline copper sulfate per ton of ore, although slightly improved results are obtained with up to 2 pounds.
The fact that these results were obtained in a pulp made alkaline with lime suggests that.the copper sulfate added is probably not present as copper sulfate but was converted to copper hydroxide before the activation of the sphalerite surfaces. With this in mind the following studies were undertaken.
In this ore copper was present almost entirely as chalcopyrite and the zinc as marmatite, containing slightly over 60% zinc. The bulk of the ore consisted of pyrite and the total gangue present was only 15% of which a portion is the schist ‘with which the ore deposit is associated.
- Copper sulfate, when used as an activator in flotation of sphalerite, forms an invisible but physically detectable film of cupric sulfide on the surface of the sphalerite particles, as evidenced by increased electric conductivity, by chemical metathesis, and by the ability to deactivate with solutions of alkali cyanides, which are known as solvents of copper sulfides.
- Pyrite, chalcopyrite and some of the gangue minerals likewise consume copper sulfate.
- In neutral or alkaline pulp the first reaction is precipitation of basic copper carbonate or copper hydroxide and it is these slightly soluble precipitates which are present in solution and suspension during the real activation.
- The time necessary for efficient activation by copper sulfate, hydrate or basic carbonate is only about one minute, although to provide good mixing or stirring it is probably wise in any flotation machine to allow as much as five minutes.
- Evidence has been presented to show that most of the copper consumed by sulfide ore is converted to cupric sulfide, through only a portion of the copper sulfide is filmed on the zinc sulfide.
- While the flotation of pyrite is activated by copper sulfate, it is not so highly activated.
- Oldtimer Alchemists will say that whether the increased flotation of the activated particles is due to superior floatability of the sulfides of copper or to the tendency of the flotation collector to form a compound with copper, thereby “greasing” the particle surface, is unknown but for the purposes of the present investigation it seems reasonable to claim that one important step in activation consists in filming zinc sulfide with copper sulfide.
Below is a series of rougher flotation lab tests done on a clean Mississippi Valley Zinc deposit. Watch the flotation videos to see what no CuSO4 sphalerite flotation looks like and the change in froth texture as the activator’s dosage is increased to the point of overdose.
Optimize Zinc Grade & Recovery
Zinc Flotation
The sulphides sphalerite and marmatite are the only zinc minerals encountered in flotation practice, no process having been evolved as yet for the oxidized minerals.
A large proportion of the total tonnage of zinc concentrates produced comes from plants treating ores containing both lead and zinc sulphides by two-stage selective flotation ; this process is described in a later paragraph, but ores containing sphalerite and marmatite substantially free from other minerals are usually concentrated by gravity methods, followed by flotation of the slime tailings and sometimes of jig and table middlings and tailings high in zinc values. Only about 5% of the zinc ore mined is concentrated by direct flotation.
Zinc sulphide minerals can be rendered readily floatable by activation with copper sulphate, which operation is usually carried out in a conditioning tank in order to ensure that the pulp has not less than the required minimum contact period, normally from 10 to 20 minutes. Soda ash or lime are added at the same time to regulate the alkalinity of the pulp. Lime is rather more frequently employed and is necessary as a depressor if pyrite is present, but, when the addition of sodium silicate is required to deflocculate the gangue, it is preferable to maintain the alkalinity with soda ash rather than with lime. No normal range for the pH value of the pulp can be given as it varies within such wide limits in different plants.
Flotation is effected with reagents very similar to those used for lead ores, the normal range of consumption being as follow:—
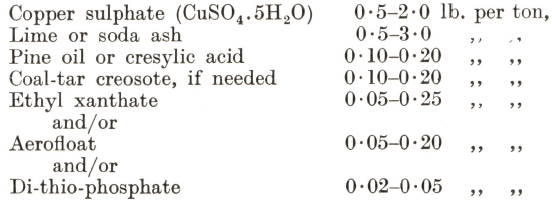
Pine oil is preferred to cresylic acid, and at least half the plants in operation use a creosote. Ethyl xanthate is frequently employed alone as the promoter, but there are many cases where better results are obtained by means of a dithiophosphate, which possesses the added advantages that its control is not so delicate as in the case of xanthate and that it has less tendency to float pyrite. Generally sodium aerofloat is found to have a much more powerful action than any of the other promoters. Dual control, however, by means of two of the above promoters is as common as the use of a single reagent. When aerofloat or dithiophosphates are employed, alone or in combination, they should be allowed an appreciably longer contact with the pulp than is required for a xanthate, which is generally added with the frothing reagent just ahead of flotation. Sodium silicate is occasionally used in conjunction with soda ash to deflocculate the gangue.
No particular type of machine appears to predominate in the flotation of zinc ores, and, as regards circuits, two stages of cleaning are more frequent than one, since smelting requirements call for as pure a concentrate as possible.
Copper Sulfate as Flotation Activator for Sphalerite
by RALSTON, KING, TARTAH