Table of Contents
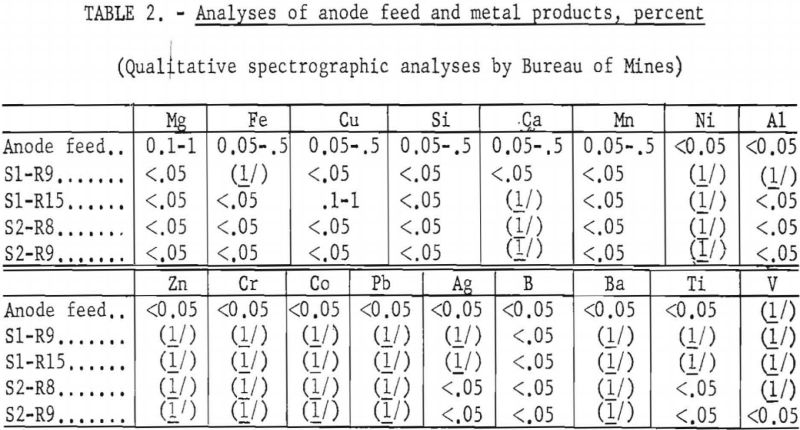
Results of the preliminary tests have demonstrated the possibility of electrorefining beryllium in KCl-LiCl-BeCl2 baths. Qualitative spectrograph analyses of the starting material and the products indicate the following estimated percentages of reduction of metallic impurities: Magnesium was reduced by at least 50 percent. Iron, copper, silicon, and manganese were reduced by at least a slight amount and possibly as high as 90 percent. Calcium was not detected in most products. Among the trace impurities, aluminum, silver, boron, and titanium still showed up in the products but at less than 0.05 percent, the lower limit of measurable detection. Nickel, zinc, chromium, cobalt, lead, and barium were not in the products. Although gaseous element contaminants were not determined for products of these tests, results obtained from similar electrorefining of titanium would indicate a reduction in such impurities. Certainly the procedure offers an encouraging route for recovery and purification of beryllium scrap and possibly for production of a high-purity metal.A brief investigation was made to determine the feasibility of electrorefining beryllium in a KCl-LiCl-BeCl2 bath. Thin, platelike crystals of beryllium metal were produced by electrolyzing technical-grade beryllium metal beads (about 94-percent purity) as the soluble anode.
Operating cell conditions were: Temperatures of 450° to 600° C.; an inert atmosphere; bath compositions of (1) 38.1 mol. percent KCl, 54.8 mol. percent LiCl, and 7.1 mol. percent BeCl2 and (2) 36.7 mol. percent KCl, 52.7 mol. percent LiCl, and 10.6 mol. percent BeCl2; cell voltages of 0.30 to 0.80; and initial cathode current densities of 50 to 435 a./sq. ft.
Current efficiencies ranging from 87 to 96 percent were obtained. Qualitative spectrographic analyses of the metal products indicated that impurities were reduced. This technique offers the possibility of producing a grade of beryllium purer than that now generally available, which may have better mechanical properties.
References to the production of beryllium metal by fused-salt electrolysis may be found in the literature: Illig and The Siemens Company published information on fluoride baths, Windecker described the working of chloride baths and Kjellgren and Tien independently reported their results on low-melting chloride mixtures. Gurklis, Beach, and Faust employed fused-salt electrolysis for refining beryllium metal.
Electrorefining may be used (1) to produce high-purity metals and (2) to reclaim scrap metals in a useful, form. High-purity beryllium metal is desired for use in the nuclear field and in metallurgical research leading to the utilization of its favorable light weight and strength in space vehicles. Reclaiming scrap in a high-purity form would greatly economize the use of this expensive metal.
The success with fused-salt electrorefining of titanium at the Federal Bureau of Mines station, Boulder City, Nev., led to the application of the fused-salt electrorefining technique to other metals. This report covers the results of the preliminary investigation of electrorefining beryllium metal. As the tests were preliminary in nature, no major effort was made to establish optimum operating conditions, and the evaluation of the products was only qualitative. Electrorefining research and the adaptation and development of analytical procedures for the metal products are being continued.
Apparatus
The apparatus shown in figure 1 was designed to permit the electrorefining operations to be performed in an inert atmosphere. The cell consisted of an electrolyte chamber and a receiver assembly, which were bolted together and sealed by a rubber gasket.
The electrolyte chamber was made from a 4-inch-diameter mild-steel pipe section, one end of which was closed by welding in a mild-steel plate ½-inch thick. The other end was equipped with a water-cooled flange. In the first series of tests the inside of the chamber was lined with a graphite crucible (not shown in fig. 1), and in the second series the chamber was not protected with a liner. The positive lead of the selenium rectifier was connected to the electrolyte chamber. Crude beryllium metal was placed
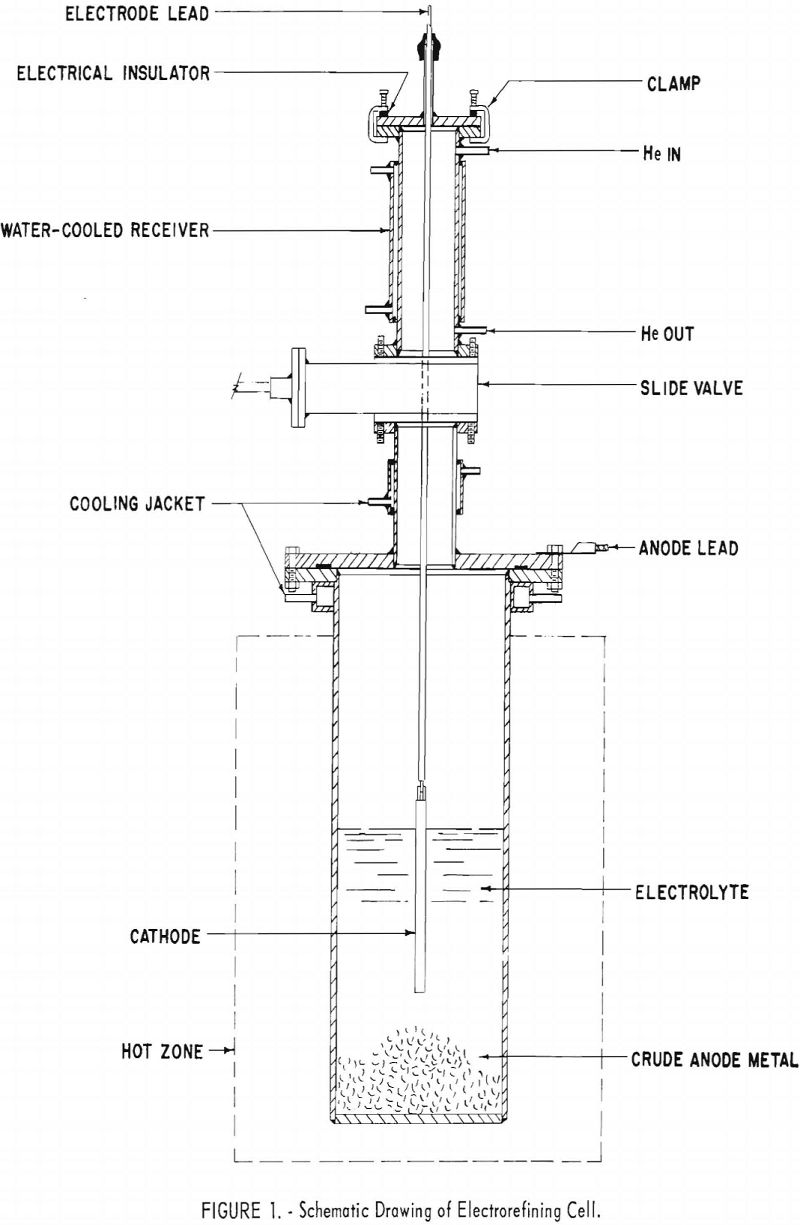
in the bottom of the bath and used as a soluble anode. The chamber was heated in an electric resistance furnace.
The receiver, which also served as a lid for the electrolyte chamber, consisted of a flange, a water-cooled slide valve, a receiving chamber, and a clamp-on cover. When desired, the receiving chamber was closed off from the electrolyte chamber by the slide valve, which was made airtight by a rubber seal actuated by two cams. The receiving chamber was water-cooled and equipped with fittings for helium, manometer, bubbler, and vacuum pump. The receiver was closed and opened to the outside, by the clamp-on cover, equipped with a rubber gasket for sealing. A fitting was provided in the clamp-on cover for inserting the cathode lead into the cell. The seal between the cover and the cathodic lead was a rubber sleeve. Electrical insulators were used at the cover clamps to prevent short circuiting when current passed through the cell. The cathode was detachable from the lead for convenience in stripping the deposited metal.
In the first series a ¼-inch-diameter molybdenum rod was used as the cathode; in the second series a 3/8-inch-diameter iron rod was used.
Experimental Procedure
The cell was assembled and tested for leaks under both vacuum and pressure. When the cell proved to be leak free, a total of 1,560 grams of c.p. (chemically pure) grade KCl and LiCl of the eutectic composition was added. The cell was then evacuated and filled with helium four times before heating to dry the salt. The salt was dried under vacuum at temperatures of 200° C. and 400° C. for a total of 24 hours. The cell, filled with helium, was then heated to the operating temperature range of 450°-600° C.
To prevent atmospheric contamination in the cell, the slide valve between the electrolyte chamber and receiver was closed whenever the receiver was opened to the outside for the removal or introduction of material during operations. Subsequently, after replacing the cover, the receiver and its contents had to be purged by evacuating and filling with helium four times before the slide valve was again opened to the electrolyte chamber.
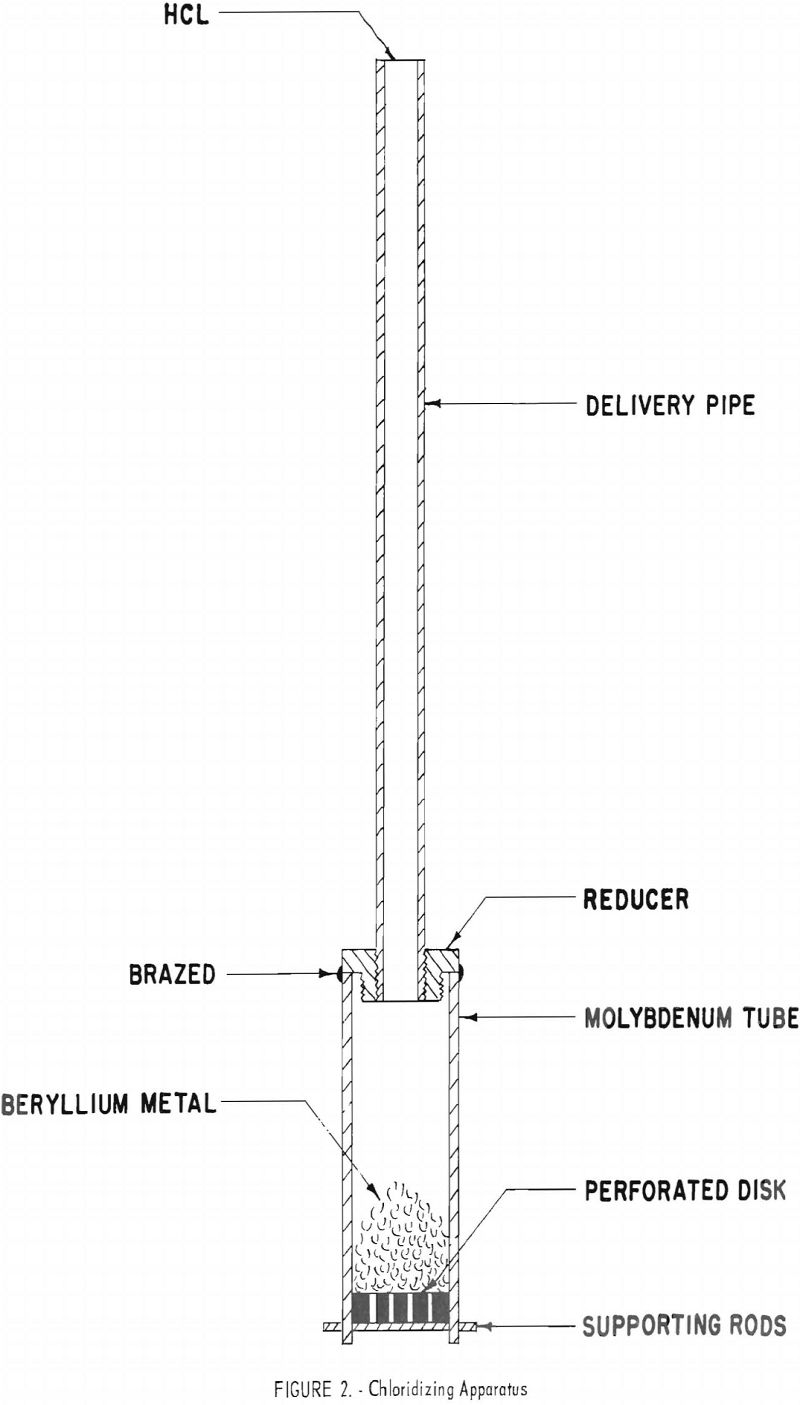
The BeCl2 component of the bath was produced in situ by chloridizing beryllium metal in a molybdenum tube inside the cell. The chloridizing apparatus is shown in figure 2. A calculated amount of beryllium metal was placed in the chloridizing tube and partially immersed in the molten salt before dry HCl was introduced for the reaction,
Be + 2HCl → BeCl2 + H2.
The BeCl2 product passed from the tube and dissolved in the molten salt, and the H2 byproduct was vented from the cell through an oil bubbler. When the amount of HCl calculated to yield the desired concentration of BeCl2 in the bath was utilized, the chloridizing apparatus was removed and the bath was sampled.
Crude beryllium metal of about 94-percent purity was added to the bottom of the bath as the soluble anode of the cell.
After the cathode, which was attached to an iron rod, had been lowered into the bath, a direct current was supplied to the cell from a selenium rectifier.
When a deposition cycle was completed, the cathode deposit was raised from the bath until the electrical circuit was broken. The entrained salt was allowed to drain from the metal deposit for 15 to 20 minutes. The deposit then was raised, sealed in the receiver, and allowed to cool. After cooling, it was removed from the receiver, and the metal was stripped from the cathode rod, leached in very dilute HNO3 solution, washed until free of chloride, rinsed with acetone, and dried in a hood.
Results
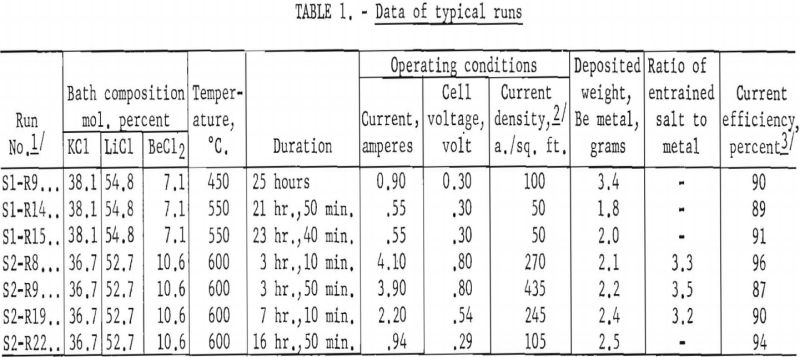
This preliminary investigation of fused-salt electrorefining of beryllium was limited to two series of tests. The first series, consisting of 18 runs over a period of 24 days, was made in a bath containing 38.1 mol. percent KCl, 54.8 mol. percent LiCl, and 7.1 mol. percent BeCl2. The second series, consisting of 27 runs over a period of 26 days, was made in a bath containing 36.7 mol, percent KCl, 52.7 mol. percent LiCl, and 10.6 mol. percent BeCl2. In all tests the highest cell voltage was 0.8, the highest amperage about 4.0, the highest cathode current density, based on the initial area of the cathode, was 435 a./sq. ft., and the bath temperatures, 450°-600° C. The cathodic current efficiencies obtained were in the range of 87 to 96 percent of the theoretical, based upon the reduction of Be++ to Be°. No significant difference in the results of the two series was noted. Data from seven typical runs are shown in table 1.
The deposited beryllium metal was in the form of long, thin, flat crystals, steel gray in color. A typical deposit is shown in figure 3. Analytical data of the feed material and refined metals (table 2) indicated that impurities were reduced and that electrorefining had taken place.
At a temperature as high as 600° C the sublimation of BeCl2 from the bath was low and inconsequential. The entrained salt with the deposited metal was leached easily with dilute HNO3 solution.