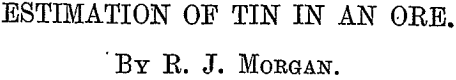Table of Contents
The object that the writer has mainly in view in presenting this paper is to draw the attention of those connected with the technical side of the industry to the unsatisfactory and by no means up-to-date methods employed in estimating the tin contents of an ore. At the same time, what is regarded, from the writer’s personal experience, as a satisfactory and reliable method is explained in detail. It is a curious fact that for the last 25 years tin-assay methods have made little or no advance, while those now in general use in connection with copper and lead ores can scarcely be compared with those then obtaining, so greatly have they been improved upon. Though it is not altogether expected that the method herein advocated will at once meet with general approval, the writer hopes the resultant discussion may bring out points that have hitherto escaped detection.
Tin Fire Assaying Methods
The old Cornish method of smelting with anthracite is now obsolete. The cyanide assay, although unreliable, is still much used because of its simplicity. In the Malaya many buyers of tin ores use the cyanide assay, the smelting being carried out generally in a benzine furnace.
It is essential that the smelting with cyanide be performed at a low temperature. Even then, impurities, such as Sb2S3 and Bi2S3, if present, are reduced, rendering the button of tin impure
Sb2S3 + 3 KCN = Sb2 + 3 KCNS
Oxides of iron are readily reduced, the iron going in with the tin, and if the button contains impurities the percentage of tin in the button must be determined by wet methods; therefore it is desirable to use a wet method, and so avoid the doubtful fusion connected with the cyanide-process.
Wet Assay Methods
In technical practice, volumetric methods are preferable, being quite as accurate and much more rapid than gravimetric methods. Consequently this paper will be confined to discussing volumetric methods. Many schemes are in use, but professional men appear to differ as to which is the most practical and satisfactory volumetric method. The two in general use at the present time are:
- Titration of stannous chloride with standard iodine solution.
- Titration with a standard solution of ferric chloride.
Both these titrations give satisfactory results, but the Writer prefers No. 1, as the operations of preparing the assay solution are simpler.
Determination Method Advocated
The following method, which is a modification of that commonly known as the Pearce-Low scheme, was found to give very satisfactory results. It is rapid, and answers every ordinary requirement:
Place from 6 to 8 gr. of stick sodium-hydroxide in a thin-spun iron crucible provided with an iron cover, and gently heat to expel moisture. Weigh out about 5 grm. of the finely-powdered ore and add to the iron crucible after the smelted hydroxide has cooled sufficiently to permit of its setting. Cover the crucible, and again heat, gently at first, and afterwards to dull redness, until fusion is complete. Remove the cover and allow the “ melt ” to cool, placing the cover in a 5¼-in. casserole, or in an ordinary porcelain evaporating basin. When the crucible has cooled, place it on its side in the casserole, add a little water, and heat to boiling point. Next, loosen the “melt” with a glass rod, wash the crucible well until all the “ melt ” has been removed, and then lift the crucible from the casserole, giving it a final rinse. Rinse the crucible with a few cubic centimetres of weak hydrochloric acid to ensure that absolutely all the “ melt ” has been removed, adding the acid to the solution in the casserole, which must be covered to prevent loss by effervescence. Remove the crucible cover from the casserole, and if any globules of the “ melt ” are still attached, remove them with a dilute acid wash.
It is important to keep the washings in the above operations as small in volume as possible.
Next add strong HCl, a few cubic centimetres at a time, keeping the casserole well covered until effervescence ceases. Then add 20 cc. of strong HCl—in excess, heat to boiling point and transfer the solution to a suitable flask. No undecomposed residue should remain in the casserole, except perhaps a little gelatinous silica. Add three horseshoe nails to the solution, the nails being bent to form the letter U. Boil for 15 minutes, and filter through glass-wool, washing the filter with about 50 cc of distilled water.
Horseshoe nails, being made of a very pure and soft iron, act efficiently in removing Cu, Sb, As, &c., and at the same time mmimize the risk of introducing other impurities.
To the filtrate, which has been caught in a 6-oz. conical flask, about 5 grm. of finely-powdered antimony is added. The solution is then heated to boiling point, and the boiling is continued for five minutes. The flask is next cooled in an atmosphere of CO2 and titrated with a standard solution of iodine, using a starch solution as an indicator. It is essential that the solution, after boiling with antimony, should be cooled as rapidly as possible, and to accomplish this the flasks can be placed in a tray and cold water circulated round them. As the stannous chloride oxidizes very rapidly to stannic chloride, especially when the solutions are hot, the solution must be cooled in an atmosphere of CO2. This may be done by placing a pea of marble in the flask This last instruction cannot, however, be recommended, as the results are generally low — probably owing to the introduction of air.
When doing a number of determinations it is better to cork the flasks during the operation of boiling, the corks being fitted with a delivery tube. On removing the flasks from the hot plate, connect the delivery tubes to a constant supply of CO2.
Alternative Determination Methods
Mr. J. J. Berringer, in an article written to the London Mining Journal, advocates the use of zinc fume or clippings for reducing the tin compounds, in place of fusion with sodium-hydroxide., The writer has used this method and found it to be an efficient one, giving results which agreed well with the method already described under the heading of “ Method Advocated.” That this is so will readily be seen by a perusal of the following table :—
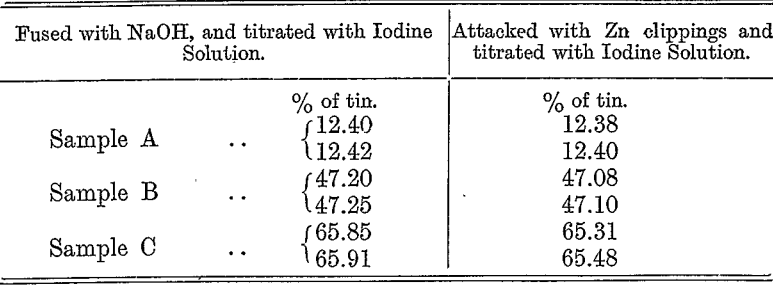
The hydrogen reduction method championed by Parry, and claimed by him to be the method is, in the writer’s opinion, cumbersome, and decidedly inferior to the above methods for accuracy, simplicity, and speed. The following table compares the results obtained by the method described in this paper with those obtained by the hydrogen reduction method:—
Methods of Reducing Stannic Solutions
Many methods are in use for reducing the stannic solutions to the stannous state ready for titration.
Iron horseshoe nails are effective if the solution is kept strongly acid and boiled for at least 25 minutes.
Sheet nickel may be used in place of iron, but it is expensive, and the strong colour imparted to the solution tends to mask the appearance of the first tinge of starch iodide, and this is objectionable when testing for small amounts of tin.
Zinc reduces tin chloride to the metallic state, and it can then be filtered off and re-dissolved in strong HCl. Experiments showed that whilst being re-dissolved there was a tendency for the tin to oxidize to stannic chloride, and in this method one is obliged to assume that the tin dissolves wholly as stannous chloride. As this assumption is not justified, the method is quite unreliable. The writer considers the best reducing material to be finely-powdered antimony, which is effective and rapid, and the excess of Sb that remains does not interfere with the iodine titration, which is performed in the cold. It is essential that the antimony should be extremely fine and free from interfering metals. Kahlbaum’s pure pulverent antimony answers well, the rapidity and completeness of reduction well repaying the cost of the pure metal.
The following table compares the efficiency of antimony, iron, and nickel as reducers :—
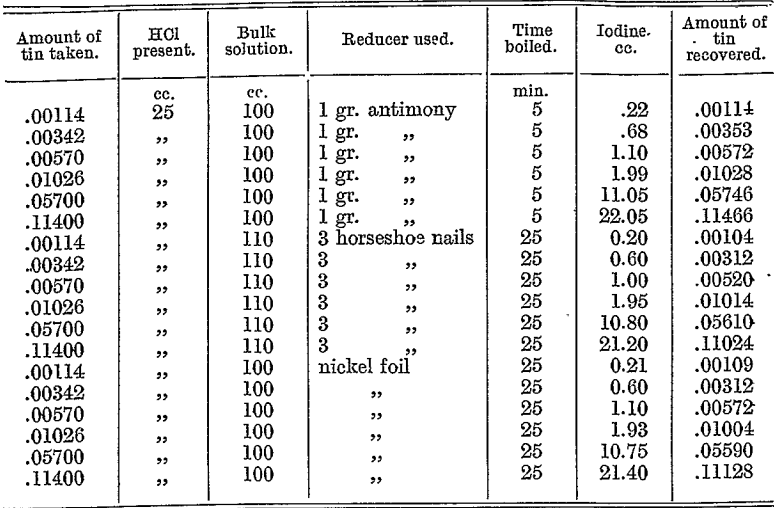
Note. With the larger quantities of tin, Fe and Ni gave low results ; only Sb reductions appear to be efficient.
Method of Titration with Standard Ferric Chloride
In the ferric chloride method of titration the strong yellow-colour of ferric chloride indicates that the conversion of the stannous chloride to the stannic condition is complete. With this method antimony cannot be used as a reducer, metallic zinc being generally employed, and, as pointed out above, this is open to error. The titration must be performed above 70° C., and at such a high temperature it is difficult to prevent oxidation.
Conclusion
After a considerable number of experiments the writer is of opinion that fusion with NaOH in an iron crucible, followed by reduction with iron nails and antimony and the iodine titration, gives the most accurate and consistent results with the minimum of trouble.
The experiments quoted in this paper were carried out at the Auckland School of Mines, under the direction of Professor Jarman, A.R.S.M. The writer is greatly indebted to Mr. M. C. Corbett, now of Mineral Separation Ltd., Perak, for assistance rendered in the experimental part of the work.
Professor A. Jarman said that Mr. Morgan’s paper was the outcome of considerable experiment by Mr. Max. C. Corbett, followed by further experiments by Mr. Morgan, who had proved himself to be a careful manipulator with keen powers of observation. The chief points of the paper as they appeared to him (the speaker) were; (1.) The old Cornish tin assay was as defunct as the dodo. (2.) The cyanide fusion assay ought to be, but is not. (3.) There was no general concensus of opinion, as yet, concerning the best wet method. He had no hesitation in saying that the fusion with NaOH in the thin iron crucible was the easiest method of attack by which to render the tin soluble. If the NaOH had been fused previously very little attention was required during the fusion and 5 or 6 fusions could easily be done at one and the same time. After the solution of the melt the preliminary reduction by iron removed Cu and As which otherwise interfered ; and after filtration there was not much reduction left to be done by powdered antimony, since all the iron salts were ferrous and a good deal of the tin was stannous. The reduction by antimony was more perfect than that by iron and very much more rapid, and with the precautions given by Mr. Morgan, it was reliable and accurate. The only point not noted was that antimony which was excessively finely ground had a slow reducing action upon SnCl4, even in the cold. The powdered pure antimony of Kahlbaum’s brand was sufficiently fine for the purpose. The titration by iodine in the cold left nothing to be desired. The method should be in general use as the results could be relied upon, but the absence of an indicator which would detect the presence of SnCl4 in a solution of SnCl2 had militated in the past against the adoption of wet methods, because of the difficulty in ascertaining when the last of the tin had been reduced to the stannous state. Powdered antimony completed the reduction in two or three minutes if a preliminary reduction by iron had been given. In the absence of that preliminary reduction antimony was still efficient, but the large proportion of ferric iron in many ores consumed the finer antimony and rendered the prelimenary reduction desirable, even if Cu and As were absent.
Antimony which was excessively finely ground had a slow reducing action upon SnCl4 even in the cold had some justification especially if the solution was very acid. However, the reaction was so very slow that it would not appreciably effect the result in the time necessary for carrying out the titration. The writer believed that if the solution were kept at the acidity stated in the paper that no reducing action would take place in the cold. In addition he (the author) would point out that the method advocated could be readily adapted for the determination of tin in a cupriferous matte. A weighed quantity of the finely powdered matte was first subjected to a sweet roast in a porcelain crucible, the roasted material being transferred to an iron crucible for fusion with NaOH.