Table of Contents
The flotation process for the concentration of ores is a method by means of which one or more of the minerals in the ore (usually the valuable ones) are picked up by means of a liquid film and floated at the surface of a mass of fluid pulp. Here they are separated from the other minerals, which remain immersed in the body of the pulp. In general, the minerals which are floated are sulphides of metallic luster, but some other minerals of metallic luster such as graphite and some sulphides with adamantine luster, such as sphalerite and cinnabar, are amenable to treatment by the process.
The importance of flotation lies in the fact that it is primarily a “slimes process” by means of which the particles of valuable mineral, too fine for efficient gravity concentration, are saved with a high percentage of recovery. Recoveries in the mills treating low-grade copper sulphide ores have been advanced 10 to 20 per cent, by the installation of the process and similar increased savings have been accomplished by the same means in mills treating sulphide ores of zinc and lead.
When finely ground ore containing sulphides mixed with a siliceous or earthy gangue is brought gently onto the surface of a body of water, in a direction forming an acute angle with the surface of the water a considerable portion of the sulphide constituent of the ore floats on the surface of the liquid, while the gangue sinks. This is the so-called film flotation, exemplified by the Wood and Macquisten processes.
When gas bubbles are introduced into a fluid pulp composed of finely ground ore and water, to which has been added (1) a small amount of certain oils, or (2) a small amount of certain acids or acid salts, or (3) a small amount of certain alkalis or alkaline salts, or (4) a small amount of a mixture of oil with acid or alkali, the sulphide particles in the ore are brought to the surface on the gas bubbles. These collect in a froth heavily laden with sulphide particles. The gangue particles are not brought up by the bubbles, but remain in the mass of the pulp. This is the so-called froth flotation. Its variations, acid-froth, agitation- froth, and pneumatic-froth processes are discussed in detail later.
The points to be explained in the operation of these processes are: (1) The flotation of solid particles in a liquid the specific gravity of which is less than that of the solid; (2) the preferential flotation of the sulphide portion of the mass; (3) the functions of the reagents used.
Froth Flotation Theory Explained
The theory here presented in explanation of the points listed in the preceding paragraph appeals to the following physical phenomena: Surface tension, adsorption, adhesion and viscosity. The first three of these are closely related.
Surface Tension Explained
Every liquid surface in contact with a gas or its vapor, behaves as if it were under tension. The value of this contractile force per unit width can be measured. Its value for water, 74 dynes per centimeter, is higher than for any other well-known liquid. (Liquid metals and fused salts are of course excepted.) This fictive tension is a convenient conception for many discussions and may be explained in terms of the intermolecular, attractions of the substances forming the boundary. Another and very useful way of considering the phenomenon is to regard each unit of surface as having associated with it an amount of potential energy which is numerically equal to the surface tension. This relation is established as follows:
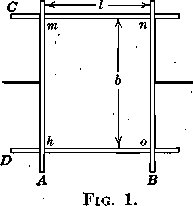
If two wires, A and B (Fig. 1), are so placed as to slide on two fixed wires, C and D, and if the wires,A and B, having been in contact, are separated a distance l against the pull of one of the surfaces only of a film, mnoh, by applying the force F, the work per unit area will be
W/A = Ff/lb = F/b = T
which is obviously equal, numerically, to the force per unit width or the surface tension, T, of the one surface considered. Applying this conception of surface energy to different cases of contact, we can develop some statements of the relations of forces which are important in explaining many of the phenomena observed in flotation.
Consider first that two blocks of liquid, which have become rigid without any change in their other properties, are drawn together by the mutual action of their forces of molecular attraction until they perfectly unite over an area A. They would do an amount of work that we will represent by the letter W. Now consider that the same two bodies when brought near together, but not into contact, become liquid and unite over the area A. The work done is again W, but in this case it can be considered as due to the shrinkage of the surface by an amount 2A, whence 2AT = W = A(T + T), where T is the surface tension of the liquid. If two different liquids whose surface tensions are T1 and T2 respectively were brought together, the work due to the molecular attractions would be (T1 + T2)A = W, provided there was complete union, but if there is not complete union, there will be an interfacial tension T12, and the energy equation becomes
(T1 + T2)A – T12A = W
or, T12 = T1 + T2 – W/A
i.e., the interfacial tension T12 is the excess of the sum of the two tensions over the work which is done by them in allowing a unit area of the two liquids to come into contact. If a liquid is brought into contact with a solid, the energy equation is Tls = TL — W1 (gas or vapor effects being excluded),
where TL = the surface tension of the liquid,
W1 = the work done in bringing a unit area of the liquid and solid into contact,
and TLS = the interfacial tension.
If, therefore, W1 = TL, the solid has the same attraction for the molecules of the liquid as the molecules of the liquid have for each other and there will be no interfacial tension. If TL > W1 the interfacial tension TLS will be positive; if TL < W1 there will be negative interfacial tension or a surface pressure. In the latter case the liquid will tend to spread over the solid.
Angle of Contact Explained:
When, as is the common case in the flotation process, there are three substances in contact, a system of forces as shown in Fig. 2 is brought into play. If O does not move indefinitely to the right or to the left, equilibrium will be attained when
TSL = TGS + TGL cosθ
cos θ = TSL – TGS/TGL
where TGS, TGL, and TSL are the interfacial tensions or pressures at the gas-solid, gas-liquid, and solid-liquid contacts respectively. From this equation is deduced the important.conclusion that as TSL increases with respect to TGS, the angle of contact θ becomes smaller (the gas and liquid being the same), or, in other words, the angle of contact is a measure of the tendency of one fluid to replace another on the surface of a solid. We have examined the angles of contact of the water-air, oil-air, and oil- water surfaces against a number of the common minerals. We have found, in general, that the air-water contact angle is less for gangue minerals than for sulphide minerals; that the air-oil contact angle is less for sulphides than for gangues and less for any given sulphide than the air-water contact angle; and that the water-oil contact with solids takes the form shown in Fig. 3.
We found further that the invariable effect of oiling a solid surface is to reduce the air-water contact angle.
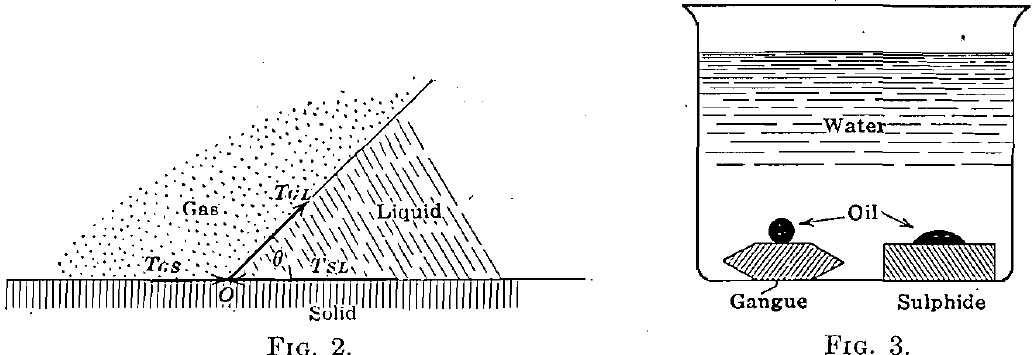
This latter phenomenon is undoubtedly aided by the reduction of the surface tension of the water due to contamination by the oil. This is further discussed later.
The conclusions forced by observation of the above phenomena are:
- That water has a smaller tendency to displace air on the surface of sulphide minerals than on the surface of gangue minerals.
- That the tendency of oil to displace air is greater at the surface of sulphide minerals than at the surface of gangue minerals.
- That oil tends to displace water on the surface of sulphides and that water tends to displace oil at the surface of gangue minerals.
- That water displaces air more readily on an oiled solid surface than on a clean surface of the same solid.
- That these tendencies toward displacement are due to the interfacial tensions or pressures existing between the various substances, and that the resulting action of these interfacial forces is a manifestation of the tendency toward reduction of the total potential energy of the system. Wherever an increase in the solid-fluid interface will decrease the potential energy, such a change will occur.
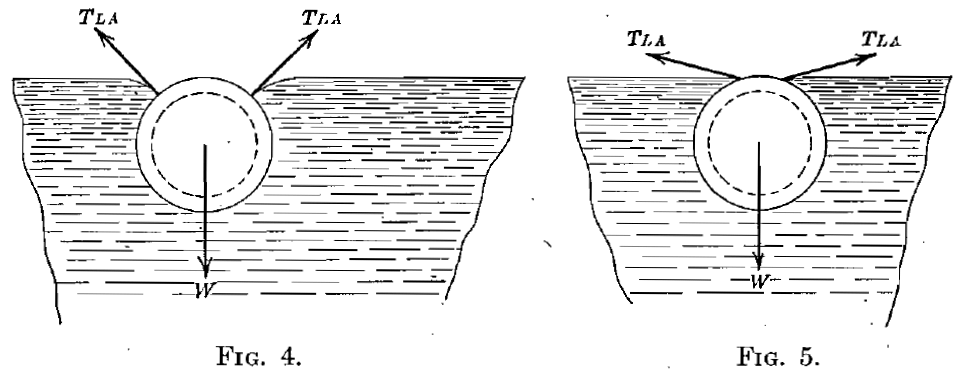
These conclusions suggested the following confirmatory experiment. A ring, 6.17 cm. outside diameter and specific gravity of 1.38, made of aluminum tubing, 0.63 cm. diameter, was cleaned and floated without trouble on the surface of pure water. The shape of the water surface at the air-water contact is shown in Fig. 4. The ring was then oiled slightly. The air-water contact angle was reduced, as shown in Fig. 5, to such an extent that it was impossible to float the ring. The same was true, as might be expected, when a cylinder of aluminum replaced the ring. A similar cylinder of glass tubing exhibited such a small air-water contact angle that it could not be floated.
Explaining Adsorption:
The surface layer between two physical phases is the seat of conditions of density and viscosity, also of apparent forces or energy manifestations, which are notably different from those in the bulk of either phase. On philosophical grounds it is impossible to consider that a real physical discontinuity occurs at the boundary between two media. In other words, there must be a very thin layer of transition in which there is a rapid but continuous change in the concentration of the components. This change in the concentration of a component at the interface is called adsorption, and may occur even between two phases which are ordinarily regarded as immiscible.
Adsorption at a gas-liquid interface may be demonstrated as follows: If a solid, which has been heated in a vacuum, is introduced into a measured volume of a gas over mercury in a calibrated tube, an amount of the gas will be adsorbed, as is shown by the change in pressure and volume compared to the space originally occupied. These additional facts are established:
- (a) The amount of the gas adsorbed at constant temperature increases with the pressure.
- (b) It is different for different gases.
- (c) It is different for different solids.
- (d) It increases as the temperature decreases.
- (e) There is an energy transformation which is indicated by the heat developed through adsorption, analogous to the Pouillet phenomena mentioned later.
- (f) Chemical reactions are assisted by the adsorbed layer.
It follows that the gas layers must vary in density, falling off rapidly with increasing distance from the solid. Quincke assumes that the density of the gas next to the solid is equal to that of the solid and concludes that the amount adsorbed will increase with the density of the solid. From these facts we conclude:
- That gases and solids exhibit selective adhesion and that, therefore, gas bubbles will attach themselves more persistently to some substances than to others.
- That this selective adhesion is a manifestation of a definite amount of energy possessed by each unit area of a gas-solid contact, and that this potential energy is capable of variation.
- That chemical reactions which diminish this potential energy are aided by adsorption.
Adsorption at a liquid-solid surface manifests itself in a vacuum, or where the vapor phase is negligible, by the way in which the liquid spreads or gathers itself together on the solid; in other words, in the way in which the liquid wets or adheres, to the solid. It is further manifested by an evolution of heat, known as the Pouillet phenomenon. A calculation of the condensation necessary to evolve this amount of heat, in the case of water against glass, indicates that the specific gravity of water in the adsorbed layer is increased to about 2.1.
Adsorption of the gas at a gas-liquid surface is indicated (1) By the effect on the surface, tension. The surface tension of a freshly formed mercury surface does not change in a vacuum, but falls off in the presence of different gases, for about an hour. Certainly the density of a liquid cannot be constant at the boundary but must go over continuously into that of the gas. (2) By the increase in the solvent power of the surface.
(3) In the case of contaminated liquids, by the concentration of one or more of the components of the liquid at the gas-liquid surface. Every unit area of such a boundary possesses a definite potential energy which always tends to a minimum. If, therefore, the surface tension of a solution depends upon the presence of any component, such a change of concentration of that component will occur as will reduce the potential energy, i.e., the interfacial tension. In other words, any component which reduces surface tension will be found in excess at the surface of a solution. For example, the surface tension of water is greater than that of alcohol. Experimentally, a drop of alcohol on a thin film of water rapidly reduces the surface tension of the water and the latter draws away from the alcohol. On the contrary, a drop of water on a thin film of alcohol spread over glass does not at once diffuse into the film hut remains gathered in a heap. Such diffusion would increase the surface energy of the system, hence the water concentrates away from the gas-liquid surface. The greater viscosity of the surface of a solution above that of the bulk, or of that of a pure liquid, has long been recognized by its damping effect upon a swinging magnetic needle and may properly be ascribed to gas-liquid adsorption. Closely connected with this is the formation of elastic solid skins or very viscous layers at a free surface, as, for example, in the case of solutions of peptone and dye stuffs. A peculiarity of saponine solution is the rigidity of its surface while the interior remains more mobile. The surface of a freshly formed fairly concentrated solution of fuchsin is quite mobile, but in the course of a few hours it changes to a reflecting skin with solid properties.
Similar results are obtained with methyl-violet and peptone. In the case of the latter substance the skin is highly elastic. There seems to be no doubt that true adsorption is present here. In the case of crystal-violet, which closely resembles methyl-violet in its properties, a solution of 1 g. to the liter lowers the surface tension from 75 to 69.9 dynes per centimeter. Other causes for the production of a solid layer may, however, be present, for many of these substances in concentrated solutions stiffen into gelatines and since the concentration of the contaminant is great at the surface and the solubility has also a different value, the solid remains persistently. Either a chemically irreversible change or a transformation into a more difficultly soluble phase at the surface is clearly the explanation of the persistence of the froth in albumen solution and the like. The properties of such surfaces apparently pass over imperceptibly into those of colloids.
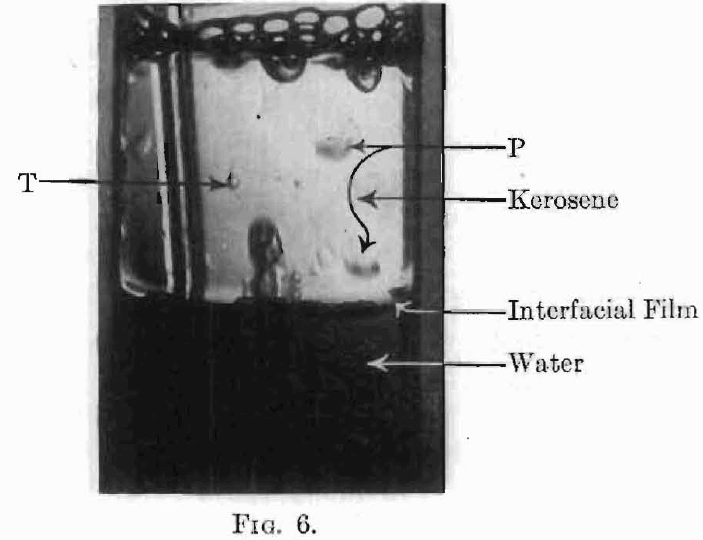
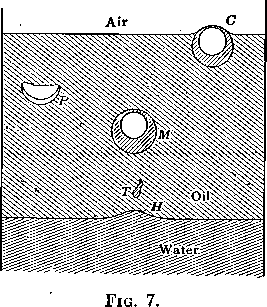
Adsorption at the boundary between two liquids is evidenced by the effect on the interfacial tension just as in the gas-liquid solution surface, although the process is not one usually described as ordinary solution and one may also have to reckon with chemical reactions in the transition layer. With liquid-liquid surfaces, as in gas-liquid surfaces, the adsorption frequently gives rise to very viscous layers. The presence of such a viscous layer at an oil-water interface is easily shown by pouring any clear oil, kerosene, liquid vaseline, etc., onto water and then bubbling a gas through the water. Such an experiment is shown in Fig. 6. The interface has all the appearance of an elastic skin. Bubbles rising through the water and striking the under side of the interface stretch the film (see H, Fig. 7), and rising farther drag away a mass of water surrounded by this viscous layer. The system now appears as shown at M and rises to the oil-air surface on account of its lower specific gravity. Here the film, together with the excess water carried up as shown at C, breaks away and falls back through the oil, not in spherical form, as would be the case were the water drop not surrounded by a viscous film, but in hemispherical form (see P, Figs. 6 and 7) often trailing behind it a film with ragged edges, as it broke from the bubble. The tadpole-shaped water drops, T, (Figs. 6 and 7) are further evidence of the high viscosity of the oil-water interfacial film.
Understand Viscosity
A marked increase in the viscosity of interfacial films is produced by the presence of finely divided solid matter. This increase is apparent in the experiment just described when finely powdered sulphide is thrown into the oil and allowed to settle to the interface, where it becomes entangled in the film. When gas bubbles are introduced, as before, the return water drops, coated with a film containing the solid particles, are much more irregular in shape than previously, and their coalescence after reaching the interface requires days or weeks. An even more convincing proof of the increase in viscosity of an interfacial film is given by the following experiment. If a needle is floated at the center of a surface of pure water in a beaker 4 in. in diameter and a chip of wood is floated near the wall of the beaker, the needle may be caused to revolve by means of a magnet without disturbing the chip. If the surface of the water is dusted over with fine ore, the whole surface together with the chip moves as though it were a rigid solid.
SUMMARY:
The potential energy at a gas-sulphide contact is less than at a gas-gangue contact; hence gas bubbles will cling with greater persistence to sulphides than to gangues. .
Oil replaces water at the surface of sulphide minerals.
Water replaces oil at the surface of gangue minerals.
Water replaces gas more readily at an oiled surface of a solid than at a clean surface.
The addition of any contaminant to water lowers the surface tension.
In any body of contaminated water there will be a concentration of the contaminant at the air-liquid surface.
Adsorption at a gas-liquid surface lowers the surface tension and increases the viscosity.
Adsorption at a liquid-liquid surface produces a film whose viscosity is higher than that of the bulk of either liquid.
The presence of finely divided solid matter in a film markedly increases the viscosity of the film.
Application to Commercial Flotation Processes Film Flotation
Pulp with or without oil or acid is fed gently, at an acute angle, onto the surface of a body of still water. The sulphide floats and the gangue sinks.
Possible cases:
- Case 1.—Sulphide, gangue, water.
- Case 2.—Sulphide, gangue, water, oil.
- Case 3.—Sulphide, gangue, water, acid.
- Case 4.—Sulphide, gangue, water, acid and oil.
Case 1. Sulphide, Gangue, Water.—The governing factor in the initial flotation of the sulphide and immersion of the gangue is the difference in the air-water contact angle with the sulphide and gangue surfaces respectively. If the difference is great, as in the case with galena and quartz, good separation is obtained. After a considerable amount of sulphide has been floated, if the surface flow is sufficiently impeded, the particles congregate into clumps by the well-known phenomenon of apparent attraction of floating particles, a scum is formed (whose viscosity and resistance to rupture are many times greater than that of the original water surface) and the floated particles are rendered more immune to immersion.
Case 2. Sulphide, Gangue, Water and Oil.—When oil is added in this process the phenomena are entirely different from the simple film flotation of Case 1. The oil concentrates at the surface since such concentration reduces the surface energy of the system. This adsorption of the oil at the gas-water surfaces causes the formation of a viscous film. When the mixture of sulphide and gangue is introduced at the surface, the sulphide particles tend to migrate into this layer and the gangue particles migrate into the water, for a sulphide particle in contact with oil represents the condition of least potential energy which is possible for the sulphide particle to assume in the system of oil, sulphide, and water. Likewise the gangue particle surrounded wholly by water represents the condition of least potential energy for the gangue particle to assume in this system. In this case also, the formation of a scum of floated sulphide increases the stability of the float.
Case 3. Sulphide, Gangue, Water and Acid.—The effects of acid are: (a) to diminish the surface tension of the liquid, (b) to diminish the gas- liquid contact angle, and (c) to increase the viscosity of the gas-liquid surface. The diminutions of (a) and (b) are more marked with gangue minerals than with sulphides. The result is that cleaner concentrate may be expected than in either of the previous cases, but at the expense of richer tailing.
Case 4.—Sulphide, Gangue, Water, Oil and Acid.—In this case a combination of results such as can be predicted from the preceding cases is obtained.
Froth Flotation
In order to explain froth flotation it is necessary and sufficient that the gas of each bubble shall be inclosed by a film of contaminated water which shall possess the following characteristics:
- Low surface tension.
- High viscosity.
- A variable concentration of the contaminant (reagent).
- A preferential adhesion of the bubble film to the sulphide mineral compared to that for the gangue minerals.
We will examine first the conditions required for the formation of a froth, or the continued existence of a thin film. Solutions which form froth are preeminently aqueous solutions and the properties of the liquid film are only secondarily determined by those of the gas.
The durability of a liquid film depends upon one or more of the following conditions:
- A low surface tension which is locally variable so as to produce stable equilibrium.
- High viscosity, which may pass over into
- Chemical irreversibility and the production of solid skins.
Pure liquids do not foam; for example, water or alcohol. The reason is obvious. As the film is thinned out by stretching or by draining away of the liquid, the surface tension is reduced at some part below the general constant value. As soon as this begins the thicker arid more powerful parts of the film drag away from the weakened parts which at once completes the rupture. These inequalities evidently would not be so marked or rapid in their operation if the surface tension were low. Increase of viscosity would also slow up the process. High viscosity and low surface tension do not occur in pure liquids. But the most important condition for durability is some means by which the equilibrium of the forces at any point in a film may be restored, when a variation of some of the forces occurs. In the case of a compound liquid or solution this is effected by the adsorption or change of concentration of one or more of the components in the film.
The surface tension of a solution is in general notably different from that of the pure solvent, and in case of water, whose surface tension is the greatest of any liquid with which we are concerned, even a minute trace of impurity is sufficient to lessen its surface tension considerably.
Consider a film of water stretched on a vertical ring of wire. If the surface tension remained constant, as it does in the pure liquid when the thickness exceeds 0.000001 cm., the weight of the lower part would stretch down the upper part until it broke. If the water contains some component whose depletion at the weaker points increases the surface tension, equilibrium will be preserved. In the stretching of a film, and in the general running away of the liquid between the surfaces of the film, which reduces the total available amount of the contaminant, such decrease of the concentration and increase of surface tension does occur, and the film remains stable under a considerable variation of external conditions. The formation of bubbles as a result of this variation of surface tension alone is well exemplified by a simple aqueous solution of soap or of acetic acid. The running out of the liquid between the two surfaces is greatly retarded by the viscosity of the liquid, a property which may be largely influenced by the surface adsorption of one or more of the components.
When, then, gas bubbles are introduced into a liquid pulp where oil is present there is formed about each bubble a liquid film whose surface tension is less and whose viscosity is greater than that of the bulk of the liquid. Some of the solid particles of the pulp move into the film and are raised to the surface with the bubble. Since there is a concentration of oil in the film, and since the diminution in potential energy at an oil- sulphide contact is greater than at a water-sulphide contact, the contaminated layer replaces the water on the sulphide surface and the sulphide moves into the bubble film, while the gangue, on which water displaces oil, remains in greater measure in the body of the pulp. The bubbles, therefore, as they arrive at the surface, carry an excess of the sulphide minerals. Upon their arrival at the surface, the bubbles of the contaminated liquid persist, owing: (1) to their lower surface tension; (2) to their ability to adjust this tension to a state of stable equilibrium; and (3) to their greater viscosity which is markedly increased by the presence of the solid particles.
Mechanical-Agitation Froth Process
Sulphide and gangue minerals are beaten up with water and oil, with or without acid, then allowed to flow into a box containing a considerable body of liquid nearly at rest. Bubbles coated with a preponderance of sulphide particles float to the surface and form a heavy, persistent froth. The gangue particles sink.
Two cases arise:
- Case 1.—Sulphide, gangue, water and oil.
- Case 2.—Sulphide, gangue, water, oil and acid.
Case 1. Sulphide, Gangue, Water and Oil.—When this pulp is beaten, air is mechanically entrapped in the form of bubbles. At the surface of every bubble in the mass there is a gas-contaminated-liquid contact which results in the adsorption at this surface of the contaminant, oil, and the production of a viscous film into which the sulphide particles, circulating in the mass, pass with a diminution in the potential energy of the system. The result is that in a very short time after the air bubble is entangled in the pulp, it is surrounded by a viscous sheath composed of an oil-water interfacial film in which are entangled,a large number of sulphide particles. The presence of the solid particles greatly increases the viscosity of the bubble sheath. When the solid-coated bubble arrives in the settling box or spitzkasten it rises to the surface. Here the bubble persists, or, bursting, transfers its load to other bubbles. This bubble persistence is due to a combination of several factors. The oils used have, in general, a slower evaporation rate than water. The tension of the bubble film is lower than the tension of a pure water bubble. The bubble has the power of adjusting itself to its tension, within limits, without bursting. The presence of the large amount of solid matter enormously increases the viscosity of the film.
Case 2. Sulphide, Gangue, Water, Oil and Acid—The addition of acid has the twofold effect of further lowering the surface tension and increasing the adhesion ratio oil solid/water-solid. The result is, in general, cleaner concentrate with or without an increase in the sulphide content of the tailing.
Heating the pulp has, in some cases, a beneficial effect. Where this is true it is probably due to (a) decreased surface tension and consequent increased stability of the froth; (b) increased number of air bubbles formed by the air released from solution; (c) in the case of viscous oils, the greater area over which oil is spread and consequently the greater number of bubbles with a viscous oil-water interfacial film; (d) probable increase in solubility of the oil and consequent greater diffusion, resulting in more widespread adsorption in bubble films.
Pneumatic Froth Process
Sulphide and gangue minerals mixed with water and oil, with or without acid, are run into a tank with a porous bottom through which air is forced. The air bubbles rise to the surface with a coating of solid particles, preponderantly sulphide, while the gangue particles sink.
The principles involved in this method are the same as explained in the agitation-froth process. The only difference is in the method of introducing air. The result of this difference is that the bubbles in the pulp are much larger than in the agitation froth method; they arrive at the surface less heavily loaded in proportion to their area; the bubble films are, therefore, less viscous, and the froth less persistent.
Potter-Delprat Process:
Sulphides and carbonates, with or without other gangue minerals, are treated with hot, dilute sulphuric acid. Bubbles of carbon dioxide and hydrogen sulphide are formed which rise to the surface with a sulphide coating and there form a froth. That part of the gangue not dissolved remains immersed. In this method as in the other froth methods, gas bubbles are formed which are surrounded by films of contaminated water, the contaminants in this case being sulphuric acid, lead sulphate, calcium sulphate and other salts formed by the action of the sulphuric acid. The films have a higher viscosity and a lower surface tension than is possessed by the bulk of the liquid. The sulphides move into the bubble films because the system composed of sulphide and this contaminated layer has a lower potential energy than the system composed of the pulp in the bulk of the liquid. The writers at first suspected that the selective action in this case might be due to preferential gas adsorption at the gas-sulphide contact, as opposed to a gas-gangue contact, but microscopic examination of mineral froths collected from the process showed that the solid particles in the froth were completely within the films and at no point in contact with gas. The persistence of the froth is due to the factors explained in connection with the other froth processes.
While the writers have made no appeal to electrostatic forces or to colloidal -phenomena in this discussion, they realize that the potential energy existing at the contact of dissimilar substances may well include electrical forces and that migration of the suspended solid particles under the influence of electrical charges, similar to the migration of colloids, may account for some of the selective action of the bubble films. But the agitation of the pulp in the mechanical- and pneumatic-froth processes and the generation of carbon dioxide gas on carbonate particles in intimate contact with sulphides in the acid-froth process, are sufficient, in their opinion, to bring every sulphide particle into contact with a bubble film. Once in contact, the preferential adhesion of the contaminated layer to a sulphide surface in the presence of water is sufficient to account for the persistent attachment of the sulphides to the bubble films, while on the other hand, the replacement of gas or oil by water on the surface of gangue particles explains the wetting and continued immersion of the latter.
The writers have a considerable bulk of experimental data on which many of the statements in the foregoing explanation are based. These, together with the data from other experiments which are projected, and photographs of many of the phenomena mentioned, they hope to present in a later paper.
An Explanation of the Flotation Process BY ARTHUR F. TAGGART AND FREDERICK E. BEACH
