Clarification of Gold Solution
The first essential to in any methods for effective Gold Precipitation is clear solution. Regardless of color, the solution must be bright and sparkling and entirely free from colloidal solids. One of the most important advantages of proper clarification is the avoidance of undue pressure build-up in the precipitate filters. With proper skill and attention it is possible to obtain such clarification with gravity sand filters, with plate-and-frame pressure filters, or even with vacuum-leaf filters.
If canvas is the filter medium, either under pressure or under vacuum, it should be stitched around the periphery of the leaf, and the stitched area painted with “P” and “B” or a similar paint. In starting a new or recently cleaned filter unit, irrespective of type, the effluent should be returned, for a few minutes at least, to the unclarified storage.
Close attention to these points will ensure a lower tail solution, less consumption of precipitant, and much more satisfactory melting and refining.
Precoating of the filter cloths with diatomaceous earth “filter aids” has been found to improve the clarification operation and to increase the useful life of the filter covers.
The precoat when properly used prevents penetration of fine slimes into the filter fabric and thereby extends the useful life of the filter cover. It also assists in removing scale-forming substances owing to the enormous surface area exposed in the precoat, so that pressure build-up in the subsequent precipitate filters from these substances is reduced. A precoated leaf can be washed clean of accumulated slimes more easily and quickly, and consequently less labor and time are required for this operation.
GOLD PRECIPITATION
Five methods of precipitating gold and silver from cyanide solutions have been used: aluminum, charcoal, sodium sulphide, zinc, and electrolytic. The first and third were specially developed for the silver ores of Cobalt, Ontario; charcoal has been used in Australia,- with some possibility of revival; and zinc, either as dust or as shavings, has been used from the beginning of the cyanide process and continues to be the standard method used throughout the world. In this section are given the technique of the processes and their application in certain mining centers.
GOLD PRECIPITATION BY ZINC
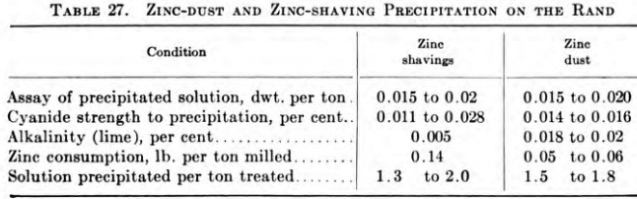
Zinc shavings and zinc dust are both used for precipitation of precious metals. Although most new cyanide plants adopt zinc-dust equipment and some old plants change from zinc shavings to zinc dust, zinc shavings probably will be used at many small mines and tailings operations. Zinc dust, however, is generally more effective and satisfactory than zinc shavings and is approximately 5 cents per ton of ore cheaper than the older method.
Chemistry of Gold Precipitation
Chemists differ somewhat on the theory of precipitation with zinc as to whether nascent hydrogen, liberated by the action of an alkali cyanide on zinc, has a direct effect in the precipitation, or is only an auxiliary action taking place at the same time. The following facts are basic:
Gold and silver are electronegative to zinc in cyanide solutions and should therefore precipitate them.
- Precipitation takes place only in the presence of free cyanide.
- Precipitation is always accompanied by the liberation of hydrogen.
- The alkalinity of the solution is increased during precipitation.
Clennell states that the entire effect of the precipitation of gold may be expressed by the equation:
KAu(CN)2 + 2KCN + Zn + H2O = K2Zn(CN)4 + Au + H + KOH
but that the reaction between zinc and cyanide takes place independently and bears no necessary proportion to it. The following equation represents the probable reaction:
Zn + 4KCN + 2H2O = K2Zn(CN)4 + 2K0H + H2
For more details see Cyanide Handbook, by J. E. Clennell; The Cyanide Process of Gold Extraction; by James Park; and Manual of Cyanidation, by E. M. Hamilton.
Preparation of Gold Pregnant Solutions for Precipitation
The requisites of effective precipitation of gold and silver from cyanide solutions are briefly as follows, based on notes supplied by the Merrill Company:
Much of the advantage of precoating is lost if the filter aid is not properly used. Attempts to “paint on” the precoat as a thick slurry of filter aid have not given good results. Thinly covered areas are not adequately protected, and a precoat which is thicker than necessary wastefully consumes filter aid. Further difficulties are experienced in attempting to replace a precoated leaf back into the clarifier tank without sloughing off part of the precoat and thereby entirely defeating the purpose of the precoating operation.
A precoat clarifier developed by the Merrill Company successfully overcomes these difficulties and has gained rather wide usage. The Merrill clarifier has a precoating arrangement which produces automatically a uniform layer of filter aid of the correct thickness on both sides of the vacuum leaf and permits return of the leaf to service without disturbing or damaging the precoat. The precoating is done in a small, one-leaf compartment usually built in at one end of the main clarifier tank and requires only simple auxiliary equipment, the main elements being a small precoating pump, a float-controlled air-solution separator, and a source of vacuum.
De-aeration or Removal of Oxygen
Efficient and complete precipitation of metals from cyanide solutions requires the preliminary removal of dissolved oxygen. The efficacy of the zinc-dust process is due largely to the preliminary removal of dissolved oxygen from the solution and subsequent prevention of reabsorption of oxygen in the solution.
The Crowe vacuum process is the most efficient and widely used method of de-aeration, since the oxygen content can be reduced rapidly from 6.5 to 0.5 milligrams per liter with a vacuum of 22 in.
A method used rather generally at one time on the Rand was to pass the gold-bearing solutions through sand clarifiers having at least 2 sq. ft. of area per ton of solution in 24 hr. To the sand clarifiers mentioned was added fine iron and highly pyritic sand. The solution was deprived of much of its oxygen as it percolated through the bed of sand, especially if two clarifiers were placed in series.
Chemical Control
For effective precipitation, solutions must contain enough free cyanide to dissolve the requisite amount of zinc and to hold in solution the compounds that are formed when zinc dissolves in alkaline cyanide solutions. This result is frequently best obtained by adding a drip of strong cyanide solution to the zinc emulsion zone when zinc dust is used.
Efficient precipitation of gold and silver solutions by means of the Merrill-Crowe process is generally independent of the strength of the solutions in cyanide and alkali. Substantially complete precipitation is obtained in some plants where solutions contain no more than 0.05 lb. of either NaCN or CaO per ton of solution. In cyaniding silver ores, solutions frequently contain as high as 5 lb. NaCN per ton, with protective and total alkali equivalent to several pounds CaO per ton solution.
In cyaniding most gold ores, the lime consumption is generally dictated by the requirements of effective settling and is almost invariably higher than necessary for the best precipitation. In cyaniding silver ores, a high alkalinity is needed to dissolve the minerals, and lime consumption for this purpose usually exceeds that needed for settling.
The principal detrimental effect of high cyanide and alkali is to consume zinc wastefully. An excess of lime in the solutions will sometimes coat the zinc and choke the filters, rendering frequent cleanups necessary.
Precipitation of all gold solutions and of some silver solutions is facilitated by the addition of a soluble lead salt to the solution. Either lead nitrate or lead acetate may be used, although the former is preferable. The amount of the lead salt approximates 10 per cent of the weight of zinc dust if this is added to the solution. The dissolved lead salt is added in the form of a continuous drip to the zinc-emulsion cone or mixing tank or may, under certain conditions, be added to the solution entering the clarifying tank but never with the zinc dust. The lead precipitates as a thin metallic film on the zinc, thus creating an active galvanic couple, with usually more rapid and complete precipitation of the gold and a lower zinc consumption. For some silver solutions the lead salt should always be added before clarification, because in most solutions some of the lead is immediately precipitated as an insoluble basic salt which rapidly clogs the precipitation filters.
The successful use of lead salts requires careful supervision because the addition of an excess at any time may coat the zinc with enough lead to retard or even prevent galvanic action. This explains why lead salts are not used in precipitating solutions containing considerable amounts of silver, copper, or lead, there being sufficient silver or base metal present to form an effective couple with the zinc.
As a precipitant activator, the Merrill Company has determined that sodium bisulphite is of practical benefit where insufficient alkaline cyanide is present. Excess alkalinity must be neutralized to about pH 6.6. One- tenth pound sodium bisulphide is required for a ton of cyanide solution.
ZINC-DUST PRECIPITATION OF GOLD
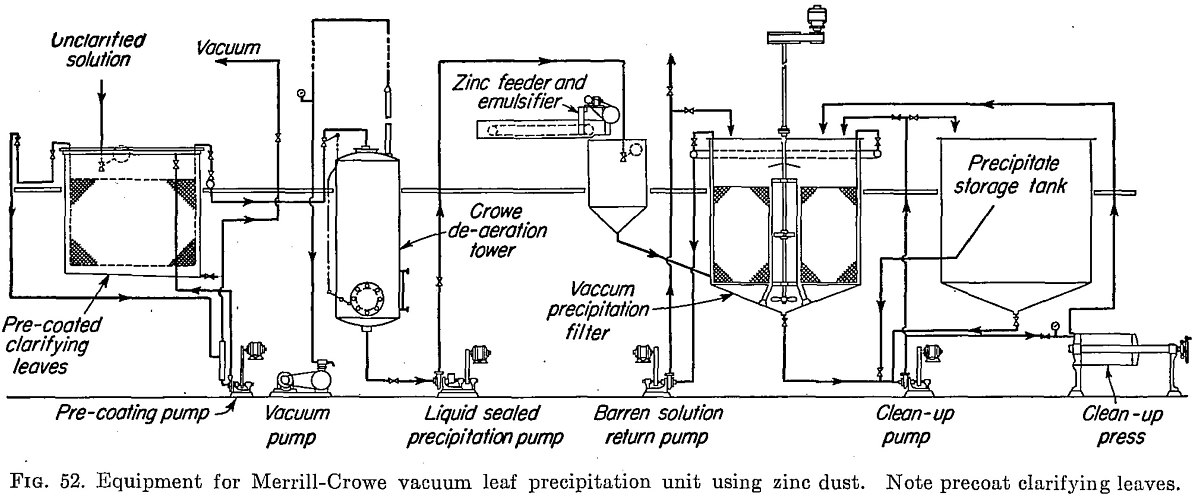
For many years precipitation by zinc dust, as by the Merrill-Crowe process, has been recognized as the most efficient and economical method of precipitating gold and silver from cyanide solutions. Embodying precipitate filters of the plate-and-frame type, of the vacuum-leaf type, or of the more recently developed pressure bag-filter type, the process is in use in the great majority of cyanide plants throughout the world (see Figs. 51 and 52).
Advantages
As compared with zinc shavings, more uniform and efficient precipitation is obtained because a fresh surface of precipitant is being constantly exposed to the solution. Where a base metal such as copper or alkaline salts such as lime, magnesia hydrates, or aluminates are present in the solution in large quantities, zinc shavings become quickly insulated or polarized, the boxes must be frequently cleaned, and fresh zinc added.
Calcium sulphate in particular is always present in the treatment of sulphide ores and rapidly coats zinc shavings, rendering them inert. In the zinc-dust process the time of contact is so short and the flow of solution through the zinc so rapid that this coating is reduced to a minimum. Even though small, unconsumed particles of precipitant may become coated with copper or sulphates, the amount of zinc thus rendered inert is negligible and is constantly replaced by the addition of fresh, active precipitant.
A difficulty frequently encountered in the precipitation of solutions containing dissolved oxygen, particularly in operating zinc boxes in relatively cold climates, is the formation of hydrated zinc oxide or so-called white precipitate. This coats and rapidly destroys zinc and, being mixed with calcium sulphate, is insoluble in acid and causes endless trouble in refining. This compound cannot form in the absence of free oxygen and is therefore entirely absent in plants using zinc dust after de-aeration.
Another reason why such uniform and efficient precipitation is obtained by zinc dust is that the filter cloths are at all times coated with a layer of fine precipitant and precipitate and no particle of solution can pass through the filter without first coming into intimate contact, in fact almost molecular contact, with the precipitating agent.
It is this extremely fine state of subdivision of the precipitant which renders zinc dust so efficient. For a given weight of metal the effective surface of zinc dust exposed is many hundred times that of shavings, and with this large area a very brief contact between the zinc and the solution is sufficient for complete precipitation of the metals.
Chemical Considerations
To obtain perfect precipitation, each molecule of metal-bearing solution must be brought into contact with a particle of precipitant, must give up its metal, and immediately thereafter must be removed from contact with other metal-bearing molecules. This condition cannot be met in a zinc box, and the resultant diffusion accounts for the long boxes necessary and the usual incomplete precipitation. In the zinc-dust process, however, this requirement is fulfilled perfectly, as the solution passes through the layer of finely divided precipitant deposited on the surface of the filter cloth.
Under the right conditions, nascent hydrogen is freely formed throughout layer of the precipitant, thus creating the reducing condition necessary for the precipitation of the metals. The actual deposition is due to the action of galvanic couples, consisting of hydrogen zinc, gold zinc, silver zinc, lead zinc, and sometimes copper zinc. The precipitation is closely analogous to electrolytic deposition, with the exception that in the zinc- dust process it is possible to obtain trace barrens because diffusion or mixing of the impoverished solution with the unprecipitated solution is prevented. Polarization of the cathode particles is minimized by the rapid flow of solution which carries the molecular hydrogen along with it.
The precipitation of gold from cyanide solutions with zinc requires either enough cyanide or enough caustic alkali or both to attack the metal with the evolution of hydrogen. Also, the zinc must be in such a form that each tiny bubble of nascent hydrogen will make contact with and adhere to a particle of zinc, forming an active couple. Obviously, this requirement is met much better within a layer of zinc powder than upon the coarse filaments of metal in a zinc box. Therefore, a much higher efficiency of the evolved hydrogen is obtained in the Merrill Crowe process than with zinc shavings. If the solutions contain dissolved oxygen, the first hydrogen generated is wasted in combining with this oxygen; this, of course, involves a corresponding loss in zinc and alkali and is entirely obviated in the zinc-dust precipitation process, which removes all dissolved oxygen from the solution before contacting with the precipitant.
Economics
The foregoing means that less zinc is dissolved per unit of gold precipitated or deposited. Less unconsumed zinc is left (10 to 15 per cent) in the precipitate; therefore melting and refining charges are less. In silver precipitation the unconsumed zinc is less, being only 3 to 5 per cent. Only 0.6 oz. Merillite or zinc dust per ounce silver is used, compared with 2 oz. when zinc shavings are used.
Most of the zinc dissolved in cyanide solutions ultimately goes to form a zinc cyanide, and each pound of zinc combines with 3 lb. sodium cyanide. Subsequently, when this solution comes in contact with fresh lime added to the ore during treatment, part of this combined cyanogen is regenerated—probably less than half, but at least 1 lb. cyanide for each pound of zinc dissolved. Hence, any method that reduces the zinc dissolved in the solutions must also be responsible for a material saving in cyanide consumption. The cleaner (less foul) solutions should also result in a higher extraction of metals from an ore.
Equipment
When first introduced, the zinc-dust process utilized the Merrill sluicing-clarifying filter for pregnant or gold-bearing solutions, the Crowe vacuum tank, a zinc-dust feeder and the Merrill triangle-shaped plate-and-frame pressure filters in which to collect the zinc-gold-silver precipitate.
Late in 1932, the Merrill Company announced a new form of equipment, the simultaneous clarification-precipitation type. This new type is now employed in capacities ranging from 100 to 1500 tons of solution daily. Many installations of this well-known equipment are in use (see Figs. 51 and 52).
Clarification and deaeration of the solution are followed by the immediate addition of zinc and precipitation of the metals without rest and without exposing the solution to atmospheric contact. Most cyanide solutions, after clarification, will, upon standing even a short time, throw out suspended colloids, consisting largely of the hydrates of alumina, magnesia, and iron. Although hardly visible to the naked eye, enough of these precipitates frequently form to coat and “insulate” the zinc, increasing the pressure in the filters and seriously interfering with precipitation. This difficulty is minimized and in most cases entirely prevented by simultaneous clarification, de-aeration, and precipitation, which cost 1 to 2 cents per ton of ore treated.
In either the bag or the leaf type of filter, a single, liquid-sealed centrifugal pump effects the successive steps of clarification and de-aeration. The clarifying tank is kept filled to a constant level with unclarified gold- bearing solution, the inflow to the tank being controlled by an automatic float valve. Suspended in this tank are the vacuum clarifying leaves, with outlets connected to a manifold, which in turn is connected to the top of the vertical vacuum tower in which the solution is de-aerated. The filter leaves after washing are immersed in the precoating compartment to which has been added a small amount of the precoat material, kept in agitation by compressed air. After deposition of the precoat layer, the leaf is returned to the clarifying compartment. Inflow of solution to the tower and, therefore, the solution level within the tower are controlled by an automatic float valve. Within the tower the solution passes down over suitable grids, which break up the flow into small streams and films, thus effecting the substantially complete removal of dissolved oxygen. The top of the de-aerating tower is connected with a dry vacuum pump which maintains a high vacuum within the tower and removes the air released from the solution. The clarified, de-aerated solution is withdrawn from the bottom of the de-aerating tower by a single-stage liquid-sealed centrifugal pump, to prevent reentry of air through the pump gland.
Where the bag precipitate filters are used, zinc dust is introduced as the solution flows from the pump to the filters. A belt-type zinc feeder, with motor drive, discharges a regulated amount of zinc dust into a mixing cone. A liquid reagent feeder, operated by the same motor drive, supplies the corresponding and uniform feed of lead nitrate solution to the cone, which is connected to the solution supply tank. The lead- zinc emulsion is withdrawn from the cone and forced into the main solution line by means of a small motor-driven, liquid-sealed centrifugal pump.

The clarified, de-aerated solution now containing the proper amount of precipitant is forced through the submerged bag filters, the precipitate of the metals remaining within the bags and the barren solution flowing over a measuring weir into a storage tank whence it is pumped for reuse.
A pressure solenoid switch is provided, which, in the event of a dangerous rise in pressure, automatically cuts out the precipitation pump. In most cases, however, operators prefer to control the pressure by a manually operated valve.
Cleanup is effected by emptying the precipitation tank, draining, and then disconnecting the bags and removing the inner filters containing the precipitate. An ordinary washing machine has proved to be quite useful in cleaning the precipitate off the bags. The precipitate is dried, fluxed, and melted in the usual way. The inner bags can be burned and added to the precipitate or washed and reused.
In the vacuum-leaf precipitate filters, the zinc dust and lead solution are similarly added to a mixing agitator, which overflows into the steady- head tank supplying the vacuum filters. The mixture of solution and precipitant is continuously circulated over the filter leaves, the barren solution being drawn through the filter leaves by a centrifugal pump, which in turn discharges to a suitable barren-solution storage tank.
The world’s largest precipitation plant is to be found at the Randfontein mine, South Africa, where the installation of 10 Merrill-Crowe vacuum filter units has a capacity for handling 20,000 tons of solution daily.
CLEANUP FOR ZINC-GOLD-SILVER PRECIPITATE
Zinc Boxes
As the operations in cleaning up the precipitate from cyanidation of gold and silver ores are so well known, little space need be devoted to them. Practice varies, but the work is relatively simple and causes little trouble. Where zinc shavings are used, generally the fine sludge is taken out of the compartments of the zinc box and later mixed with that from washing the long zinc. The sludge may be acid-treated and then washed, dried, roasted, and fluxed before melting, or it may be only dried and fluxed before melting. At some plants the entire contents of the zinc boxes are acid-treated at every cleanup, but this is not advisable because it entails considerable labor, and as only new zinc is added to the boxes, proper precipitation does not start so quickly as when at least half of the cells are filled with old zinc. When zinc shavings are used for precipitation, less than 60 per cent of the gold and not more than 75 per cent of the silver precipitated are recovered at any one cleanup, the remainder being returned with the old zinc to the boxes.
Where a silver ore is treated by the cyanide process, there have been some notable improvements in the method of emulsifying and adding the zinc dust for precipitation. Early practice was to emulsify the dust in a portion of pregnant solution and to inject this emulsion by means of a triplex pump into the pregnant flowing in the pipeline to presses. The first improvements came many years ago with the substitution of barren solution into which the zinc dust was emulsified and the replacement of the triplex pump with a small multistage centrifugal. The most recent improvement has been to emulsify the zinc dust in water, which greatly increases its precipitating efficiency, and to use a Shriver high-pressure diaphragm pump to inject into the stream of pregnant solution.
Filter Presses
Cleanup of the filters used in the Merrill-Crowe process is much simpler and quicker. The precipitates from the filters are uniformly high in gold and silver and in many instances are weighed, fluxed, and melted without preliminary treatment. In most plants the bullion is ready for shipment within 8 hr. after cleanup of the filters is begun.
On gold ores, the precipitates assay 60 to 90 per cent bullion with as little as 5 to 10 per cent total zinc, which in most plants is melted direct. Some operators prefer to give a muffle roast before melting, and in a few of the larger plants acid treatment is still used. In such plants, precipitates may be pumped from the precipitating tank direct to the acid-treatment tank, without intermediate handling.
In large plants the precipitate, either with or without acid treatment, may be melted with litharge, and the resultant lead cupeled, the bullion by this method being of much higher grade. In general, the cupellation method is to be recommended only where large amounts of gold bullion are produced.
In precipitating silver solutions, particularly when using Merrillite as a precipitant, the raw precipitate when taken from the filters contains 75 to 94 per cent pure silver, and this product is, of course, suitable for fluxing and direct melting without preliminary treatment. The moisture in the precipitate is sometimes reduced to 15 or 20 per cent before melting, but this is not necessary, particularly in the large stationary reverberatory furnaces used in the larger silver mills.
An important point in favor of this process and one that should appeal particularly to operators of customs works is the ability to clean up and convert into bullion at any time all the precipitated metals in the plant. Furthermore, the uniform ratio of precipitant to bullion and the fact that a complete cleanup is made make it possible to check accurately the bullion against both mill heads and residues and against solution assays, all of which makes the detection of theft a relatively simple matter.
ZINC-SHAVING for Gold PRECIPITATION
The older method of precipitation of gold on zinc shavings is still used in certain districts. One feature of zinc-box precipitation is that the whole operation can be seen at a glance—solution flow, effect of lead salts, whether copper is coming down, formation of zinc white, and generation of hydrogen. Precipitation on shavings is efficient and reasonably low in cost, but it offers chemical and manual problems not arising in precipitation on zinc dust.
Copper and all its compounds readily dissolve in cyanide solutions and form an adherent deposit on the zinc. The unsuspected presence of copper in an ore is revealed by zinc shavings’ becoming red.
Precipitation on zinc shavings is sometimes hindered or prevented by the formation of so-called “zinc white,” the cyanide and hydrate of zinc. It is insoluble in water but soluble in cyanide solutions and acids.
Precipitation at Kolar
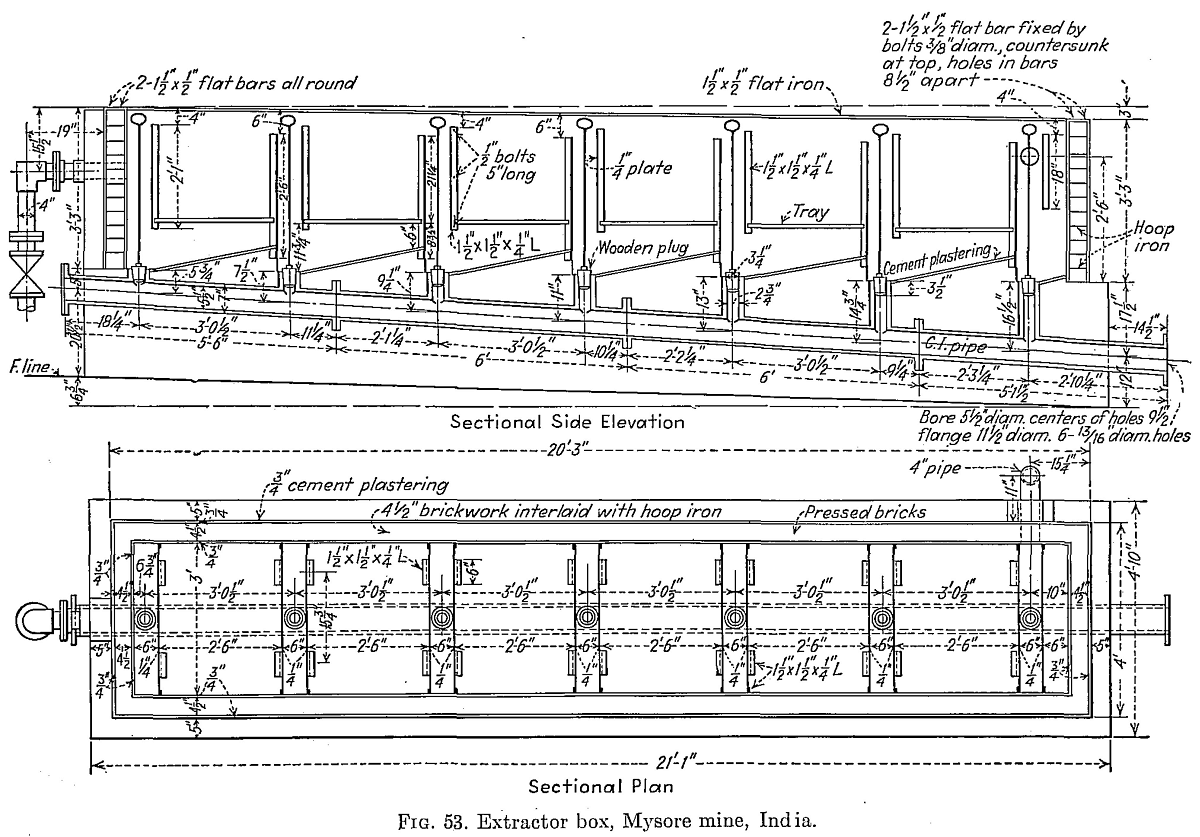
At Kolar, India, the group of mines is still using zinc shavings for precipitation, the shavings being cut locally; all solutions are clarified. A typical plant has three zinc boxes with six compartments each (Fig. 53), five of which are used. Each compartment has a capacity of 12½ cu. ft. or 187½ cu. ft. in 15 cells. A total of 620 tons is precipitated in 24 hr. Average solution feed assays 36 grains gold per ton. Of this 92 per cent is precipitated in the first two cells, and gold is rarely found below the fourth cell. Fresh zinc is dipped in a solution of lead acetate. Zinc consumption is 0.112 lb. per ton of ore treated. Most of the zinc boxes are built of concrete. Box compartments are connected by branch pipes to a main sublevel pipe or side launder discharging into a vacuum-filter tank. Wooden plugs in each cell control the flow to the vacuum-filter tanks when cleaning up. The cleanup proceeds along standard lines and is done two or three times per month. Between cleanups the zinc boxes are rarely dressed. Precipitate is treated with sulphuric acid, roasted, fluxed, and smelted. The bullion averages 985 fine.
Precipitation on the Rand
Zinc shavings and zinc dust are both used to precipitate gold on the Rand, all new plants using the latter.
De-aeration of pregnant solution for zinc shavings has. eliminated the white precipitate of hydrated zinc oxide. Lead salts are added before precipitation. Wartenweiler, in Trans. 112, A.I.M.E., 1934, summarizes the two methods as follows: During 1933, 1,055,000 lb. zinc- dust and 3,129,000 lb. zinc shavings were consumed.
Gold PRECIPITATION BY ALUMINUM DUST
Precipitation of the precious metals from cyanide solution by aluminum differs from the precipitation by zinc in that aluminum does not replace the precious metals in the cyanogen compound. In the case of zinc the reaction may be expressed by the equation:
2NaAg(CN)2 + Zn = Na2Zn(CN)4 + 2Ag
(Park, The Cyanide Process, p. 180, 5th ed.)
or, NaAg(CN)2 + 2NaCN + Zn + H2O = Na2Zn(CN)4 + Ag + H + NaOH
(Clennell, The Cyanide Handbook, p. 123, 2d ed.)
When aluminum is used, Moldenhauer, who patented this method in 1893, suggested the following equation:
6NaAg(CN)2 + 6NaOH + 2Al = 6Ag + 12NaCN + 2Al(OH)2
the aluminum hydroxide dissolving in an excess of caustic to form sodium aluminate:
2Al(0H)3 + 2NaOH = Na2Al2O4 + 4H2O
Hamilton (Manual of Cyanidation, p. 190)
suggests that the following may represent more nearly the actual reaction based upon plant observation:
2NaAg(CN)2 + 4NaOH + 2Al = 4NaCN + 2Ag + Na2Al2O4 + 4H
It is seen that the presence of caustic soda is essential when aluminum is used. Furthermore, as a matter of practical operation lime must be absent at the time of precipitation; otherwise the following reaction will take place:
Na2Al2O4 + Ca(OH)2 = CaAl2O4 + 2NaOH
The calcium aluminate so formed would contaminate the silver precipitate and result in a low-grade product extremely difficult to flux and melt into bullion.
After precipitation, however, when the barren solution is reused in the grinding and agitation circuits in the presence of lime, the aluminum is precipitated as calcium aluminate and removed from the plant with the tailing, caustic soda being formed.
At the Nipissing mill where only a small amount of lime was required and where the solution was already high in caustic from the preliminary desulphurizing process, no trouble was experienced with the formation of calcium aluminate in the press.
In order to overcome the ill effects of lime in aluminum precipitation when treating ores where the use of a fairly high amount of lime is necessary to promote effective settling, Hamilton and Crawford devised a treatment at the Butters Divisadero mine based upon the following reactions:
Ca(OH)2 + Na2C03 = CaC03 + 2NaOH
CaSO4 + Na2C03 = CaC03 + Na2S04
(Hamilton, Manual of Cyanidation, p. 195)
It allows the use of all the lime necessary for neutralizing and settlement; it yields a lime-free solution, for precipitation, and incidentally manufactures the caustic soda necessary for that operation.
Apparently, aluminum dust is not effective as a precipitant for gold alone, although the gold in solutions which contains 2 oz. silver or more per ton is almost completely precipitated. It has proved its advantages in the treatment of certain silver ores containing arsenic and antimony. It has not been widely applied, however.
Gold PRECIPITATION BY SODIUM METABISULPHITE
The practice of gold precipitation by sodium metabisulphite was developed in 1916 at the Nipissing mill to replace aluminum precipitation. The change was necessary because of the changes in economic conditions wrought by the First World War.
The sodium-sulphide process involves the precipitation of the silver as silver sulphide, the reduction of the precipitate to metallic silver by a desulphurizing treatment and the melting down of the resultant silver to a fine bullion.
At the Nipissing, gold precipitation was effected in two wooden tanks, 5 by 6 ft., provided with mechanical agitation. In the first tank the clarified solution met a small stream of concentrated sodium sulphide, which threw down the silver sulphide as a fine precipitate. To avoid blinding the canvas, the precipitate was caused to agglomerate by agitating it in a second tank before it was drawn off to the filter press. In practice it was found that 0.06 lb. sodium sulphide (60 per cent strength) was required to precipitate 1 troy oz. silver. Gold was not precipitated at all, nor was copper, if the solution contains 0.15 per cent or more free cyanide.
As with aluminum, gold precipitation by sodium sulphide regenerates all the cyanide combined with the silver in the pregnant solution. The reactions involved are shown in the equation:
2NaAg(CN)2 + Na2S = Ag2S + 4NaCN
At the Nipissing the precipitate was reduced to metallic silver by a modified form of the Denny desulphurizing process. The precipitate of silver sulphide was transferred to a 7- by 5-ft. iron tank provided with a mechanical agitator. Aluminum ingots weighing 500 lb. were thrown in, and caustic soda was added, about 0.03 lb. 76 per cent NaOH being required for each ounce of silver. With a dilution of 4 to 1 the solution had a strength of about 8 per cent NaOH. The mixture was agitated until the black silver sulphide turned brown. This required about 8 hr., depending upon the temperature of the solution. The caustic solution could not be too hot, as the reaction with the aluminum would then become too violent. This would interfere with the reduction of the silver sulphide, as the large amount of hydrogen given off prevented the actual contact necessary between the sulphide and the aluminum. The desulphurized precipitate was collected in a filter press, washed free of sodium sulphide, and delivered to the refinery. The aluminum ingots remaining were left in the bottom of the tank for the next charge.
Sodium Sulphide Precipitation of Gold
Sodium sulphide has from time to time been suggested as a precipitant for silver and it should be a highly efficient reagent where silver alone is to be considered. The difficulty is that it rarely happens that the amount of gold in a silver ore is small enough to be negligible and as gold is not precipitated by sodium sulphide the method would have to be supplemented by some other process in order to recover the gold. The advantages of sodium sulphide for silver precipitation are its low cost and its faculty of regenerating the combined cyanide, thus, 2KAg(CN)2 + Na2S = Ag2S + 2KCN + 2NaCN. Ordinarily it would produce a very pure precipitate because copper is not thrown down and about the only metals likely to be precipitated with the silver would be lead and zinc if dissolved from the ore. Its drawbacks are that it does not recover any gold that may be present and that the resulting product is not silver but the sulphide of the metal, which is not so readily converted into bullion. The process is now in use at the Nipissing Mill with apparently satisfactory results having displaced aluminium dust when the price of the latter reagent became prohibitive under war conditions. The resulting silver sulphide is reduced to a metallic slime by the Denny desulphurizing process which incidentally regenerates most of the sodium sulphide used in precipitation.
PRECIPITATION of GOLD ON CHARCOAL
T.P. 378, U.S.B. of M, 1927, by John Gross and J. W. Scott, is a most comprehensive publication on the use of charcoal as a precipitant. It lists 93 references to the literature since 1891 and 7 between 1830 and 1890. Although these items are scattered, doubtless charcoal has been most used in Australia, where, at certain times and in certain interior places, zinc has been expensive. That condition no longer exists, for Australia now produces all the zinc shavings and dust required. Gross and Scott briefly review what had been done prior to their research in about 1926 and then detail their many careful experiments. Their findings are as follows:
- The mechanism of the precipitation involves adsorption accompanied by a chemical change.
- Precipitation of silver on charcoal from cyanide solution follows the same laws as precipitation of gold, although it is slower; charcoal has less capacity for silver than for gold.
- The limit of charcoal precipitation from cyanide seems to be about 2000 oz. of gold and 1000 oz. of silver per ton of charcoal.
- Little difference exists among charcoals prepared from different woods.
- The most important point in the making of charcoal is the heat treatment, either during the making or subsequent thereto.
- To quench charcoal does not improve it.
- Pulverization finer than 200 mesh does not appreciably add to the efficacy of charcoal.
- Few substances in the solution appreciably affect precipitation.
- The adsorbed gold or silver salt is soluble to some degree in boiling water and is especially soluble in hot cyanide.
- There is a possibility of so changing the adsorbed gold or silver salt on charcoal that the charcoal may be used for further precipitation.
- Precipitation of gold on charcoal from cyanide is not metallic and has not the chemical properties of the metal. No gold is visible, even when observed under the microscope.
- Few substances in solution have a bad effect on precipitation of gold or silver on charcoal, but sodium sulphide and free cyanide decrease the rate.
- There is a loss of cyanide in charcoal precipitation, due to adsorption.
- Precipitation of gold is effective from low-grade solutions, but silver is slower, and a countercurrent method is proposed.
- Some regeneration of cyanide is possible from charcoal when sodium sulphide is used as a “fixer.”
- Charcoal could replace zinc when foul solutions cause trouble in precipitation.
- A small, isolated plant having wood available could employ charcoal in preference to zinc, using three stages with fairly coarse charcoal.
- The charcoal has to be burned, and to avoid loss by dusting in doing this, it could be impregnated with sodium carbonate.
- Charcoal will precipitate gold or silver from a cyanide-ore pulp; the charcoal can then be separated from the pulp by flotation.
With regard to findings 1, 9, and 10 wherein “adsorption” and “adsorbed gold” are mentioned, Gross and Scott credit A. W. Allen for advancing the theory that adsorption without chemical change of the alkaline aurocyanide was the correct explanation. This was generally accepted, and all known facts seemed to bear him out. Allen’s discussion is to be found in Trans. I.M. and M., 1917-1918, in Vol. 18 of M. ancl C.E., 1918 (now C. and M.E.) in Vol. 106 of E. and M.J., 1918 and in Julian and Smart’s Cyaniding of Gold and Silver Ores.
The use of charcoal as a precipitant preceding flotation is covered by Australian Patent 15,635, and for more recent developments along the lines of charcoal precipitation, the reader is referred to the carbon-cyanidation process.
