Table of Contents
Hot carbonate purification will remove hydrogen sulfide from gas mixtures of low or high concentration of this component. The presence of some carbon dioxide in the feed gas is necessary for regeneration of the solution. Carbon dioxide aids in decomposing the bisulfide and retards formation of potassium sulfide.
Carbonyl sulfide is hydrolyzed by the hot carbonate solution, and a high degree of removal of this component results. The rate of removing hydrogen sulfide and carbon dioxide is accelerated by Increased temperature of the carbonate solution. The rate of absorption of hydrogen sulfide is about 30 times faster than that of carbon dioxide at room temperature, and about 13 times faster at 110° C. Selective removal of hydrogen sulfide is favored by short contact time.
Regeneration of the fouled solution containing hydrogen sulfide and carbon dioxide proceeds readily. Although the steam consumption for regenerating solutions containing hydrogen sulfide was not thoroughly investigated, the data indicate that no more (probably less) steam is required than for regenerating the same amount of carbon dioxide between the same limits of conversion. Where almost complete removal of hydrogen sulfide is required, the bulk of the hydrogen sulfide is removed at elevated temperature, and the final amount is removed at lower temperature with a small quantity of highly regenerated solution, possibly followed by make-up solution.
Because the solubility of synthesis gas and methane in hot carbonate solution is very low, there would be little loss of these components during purification.
Results of studies by the Federal Bureau of Mines on a pilot-plant scale for removing hydrogen sulfide and carbonyl sulfide from gas mixtures by the hot carbonate system are given in this publication.
Gases containing up to 10 pct hydrogen sulfide and 48 grains carbonyl sulfide per 100 cu.ft. were treated. In most tests carbon dioxide also was present and was removed simultaneously with the sulfur compounds. Because the rate of absorption of hydrogen sulfide is much more rapid than that of carbon dioxide over a wide temperature range, selective removal of hydrogen sulfide occurred in the bottom section of the absorber near the point of gas entry. Hydrogen sulfide was removed to pipeline specifications with carbonate solutions by using split-stream flows in the absorber and regenerator.
Synthesis gas and hydrogen, produced by the partial oxidation or reforming of cual or other fuels with steam, usually require purification to remove carbon dioxide and sulfur compounds. Hydrogen sulfide and quantities of organic sulfur compounds are formed when the fuel contains sulfur.
In the synthesis of hydrocarbons by the Fischer-Tropsch reaction, costs of gas purification are high because of the amount of carbon dioxide that must be removed and the extremely low allowable concentration of sulfur in the gas (less than 0.1 grain per 100 cu.ft.). Consequently, the Coal-to-Oil Branch of the Bureau of Mines at Bruceton, Pa., in its studies on synthesis and related steps, developed an efficient process for purifying synthesis gas at elevated pressure with a hot solution of potassium carbonate. Results of pilot-plant tests dealing principally with removing carbon dioxide have been published and several commercial plants have been constructed for purifying natural gas, ammonia synthesis gas, and hydrogen by using the hot carbonate process.
Additional information regarding pilot-plant studies is given in this paper for tests with feed gases containing hydrogen sulfide, both with and without carbon dioxide, and for a few tests where carbonyl sulfide was added to the feed gas. Data is presented on the effect of temperature on the rates of absorption of hydrogen sulfide, carbon dioxide, and carbonyl sulfide in a solution of potassium carbonate, equilibrium pressures of hydrogen sulfide over the hot solution, gas solubilities, and corrosion.
Rates of Absorption
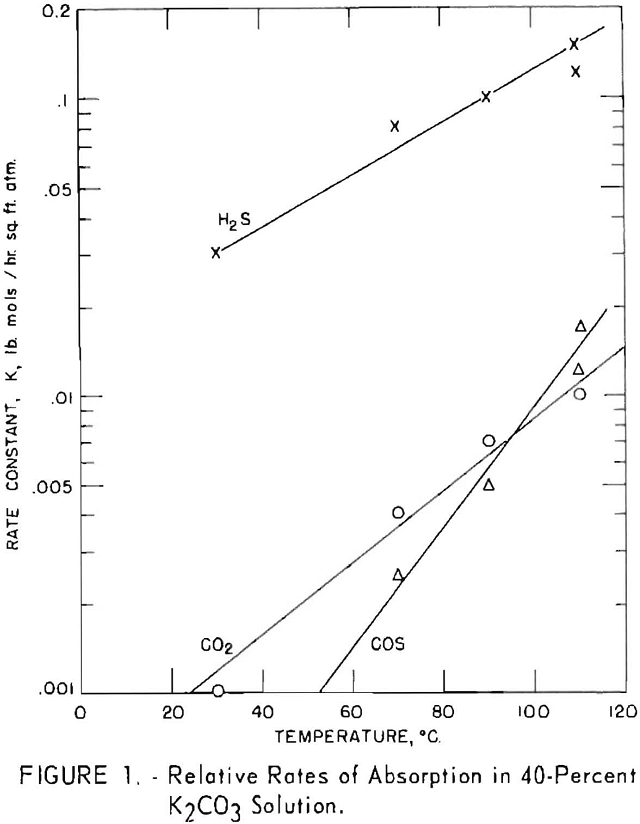
A series of batch tests was made to determine the effect of temperature on the rates of absorption of hydrogen sulfide, carbon dioxide and carbonyl sulfide in a solution of potassium carbonate. A gage glass fitted with a stirrer was placed in

an oil bath that was thermostatically controlled to within ± 0.5° C. This was used for the determinations. A fixed amount of a 40-pct. solution of potassium carbonate was charged to the gage glass, brought to desired temperature, and then evacuated to remove air from the system. The test gas was added rapidly above the liquid until 30 p.s.i.g, was reached. The stirrer then was started, and pressure readings were taken at timed intervals to measure the rate of pressure decline. Empirical-rate constants K (lb. moles/sq.ft.-hr. atm.) were determined from the time-pressure plot. The internal cross-sectional area of the gage was used as the interfacial area between the solution and gas. Figure 1 indicates that the rate of absorption increased as temperature increased. The variation of log K with temperature was linear for the three gases, but the effect of temperature on the carbon dioxide- and especially on the carbonyl sulfide-was more pronounced than on the hydrogen sulfide. Hydrogen sulfide was absorbed at a much higher rate than the other gases; the rate at 30° C. was 30 times that of carbon dioxide; at 110° C. it was about 13 times greater. Carbonyl sulfide is not absorbed as such but is converted by hydrolysis according to the equation
COS + H2O → CO2 + H2S,
and this reaction is accelerated sharply by higher temperatures. Consequently, the disappearance of carbonyl sulfide was negligible at 30° C., but at 110° C. its rate of absorption exceeded that of carbon dioxide.
Equilibrium Data
The equilibrium pressure of hydrogen sulfide over a solution of potassium carbonate depends upon the following:
- H2S content of the solution.
- Conversion of the solution to bicarbonate and bisulfide.
- Temperature.
- Concentration of the solution.
A limited study was made of the effect of these factors on the equilibrium pressure of hydrogen sulfide in the system composed of K2CO3-KHCO3-KHS-H2S-CO2-H2O. Typical data showing the effect of the aforementioned factors are given in figures 2 to 7. A steam-jacketed, rocking autoclave was charged with the dry potassium salts (carbonate and/or bicarbonate) and was evacuated. The desired quantities of water and hydrogen sulfide then were added, and the autoclave was rocked while being heated to the desired temperature and attaining equilibrium. Gas samples at equilibrium pressures were collected in evacuated containers, and the sample pressure kept below 20 mm. Hg to prevent condensation of water vapor. Concentrations of hydrogen sulfide to 0.5 pct. were analyzed by the methylene-blue method those above 0.5 pct., and all concentrations of carbon dioxide and water vapor, were analyzed by a mass spectrometer.
Figure 2 shows the pressures of hydrogen sulfide over a 30-percent equivalent solution of potassium carbonate at various hydrogen sulfide contents and temperatures of 70°, 90°, 110°, and 130° C. (The “equivalent concentration” of a solution is the concentration that would exist if all potassium salts in the solution were present as carbonate. Thus, a 30-pct. equivalent solution is always a solution that would contain 30 g. of potassium carbonate and 70 g. of water if all the bicarbonate in the system were converted to carbonate. A 30-pct. potassium carbonate solution completely converted to bicarbonate contains no K2CO3, 39.7 percent KHCO3 and 60.3 percent H2O.) In this group of tests only potassium carbonate was placed in the autoclave. The hydrogen sulfide concentration in the solution ranged from 0.6 to about 4.5 cu.ft. /gal. This is enough to convert from 7 to 54
pct. of the original potassium carbonate. A gallon of 30-pct. solution is completely converted by 8.35 cubic feet of hydrogen sulfide.
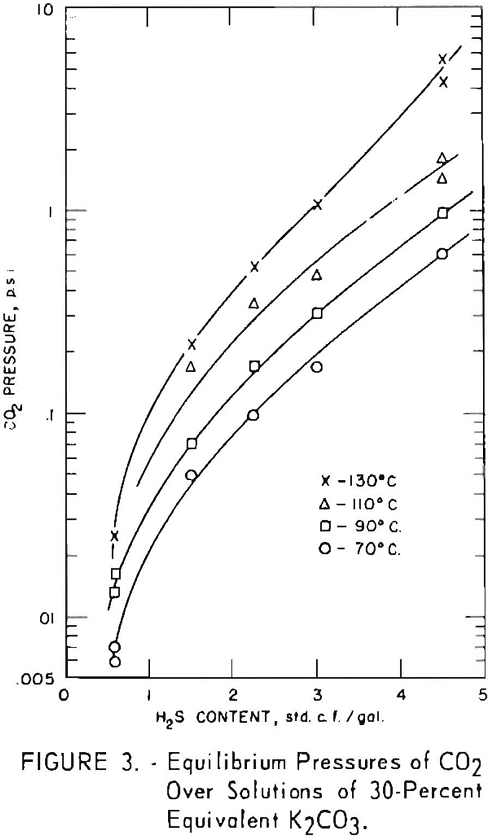
Although no carbon dioxide was charged into the autoclave in these tests, considerable carbon dioxide was present at equilibrium as gas above the liquid, as shown in figure 3. This carbon dioxide came from decomposition of part of the potassium bicarbonate formed by the reaction of hydrogen sulfide with potassium carbonate according to the equation
K2CO3 + H2S → KHCO3 + KHS.
Total equilibrium pressures of hydrogen sulfide and carbon dioxide over the solutions are shown in figure 4 for the same tests shown in figures 2 and 3. For solutions containing at least 1 standard cubic foot per gallon at 130° C., the partial pressure of carbon dioxide exceeded that of hydrogen sulfide; at 110° C. they were approximately equal, and at the lower temperatures the partial pressure of hydrogen sulfide was greater.
In continuous purification, some loss of carbon dioxide from the solution occurs during regeneration, and a corresponding amount of carbonate is depleted, forming potassium bisulfide. Potassium bisulfide is not readily regenerable in the absence of bicarbonate thus carbonate solutions are probably not suitable for removing hydrogen sulfide from gases free of carbon dioxide. As long as enough carbon dioxide is scrubbed from the impure gas to replace that lost in the regeneration, carbonate can be used effectively for hydrogen sulfide removal.
In figure 5 the equilibrium pressures of carbon dioxide are compared with those of hydrogen sulfide above 30-percent potassium carbonate solutions at 110° C. containing each gas alone.
In all cases between 70° and 130° C., for a given amount of acid gas in solution, the equilibrium pressure of
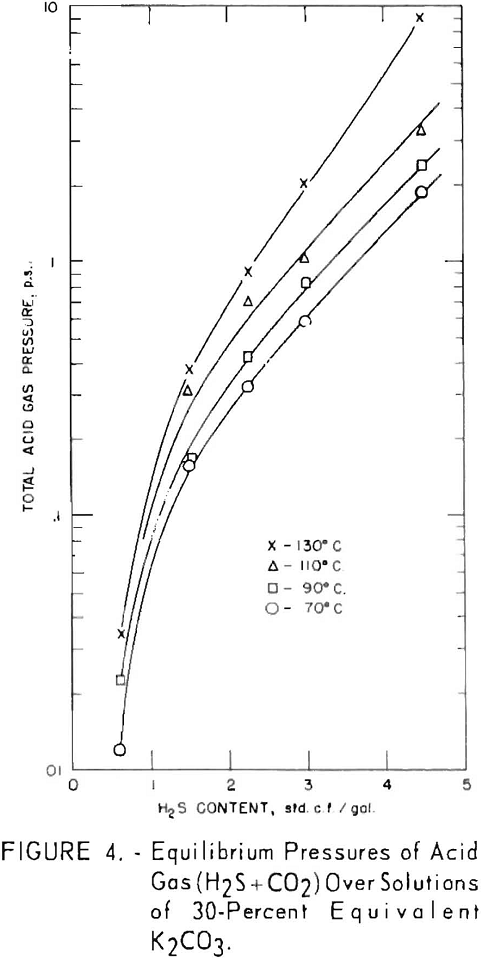
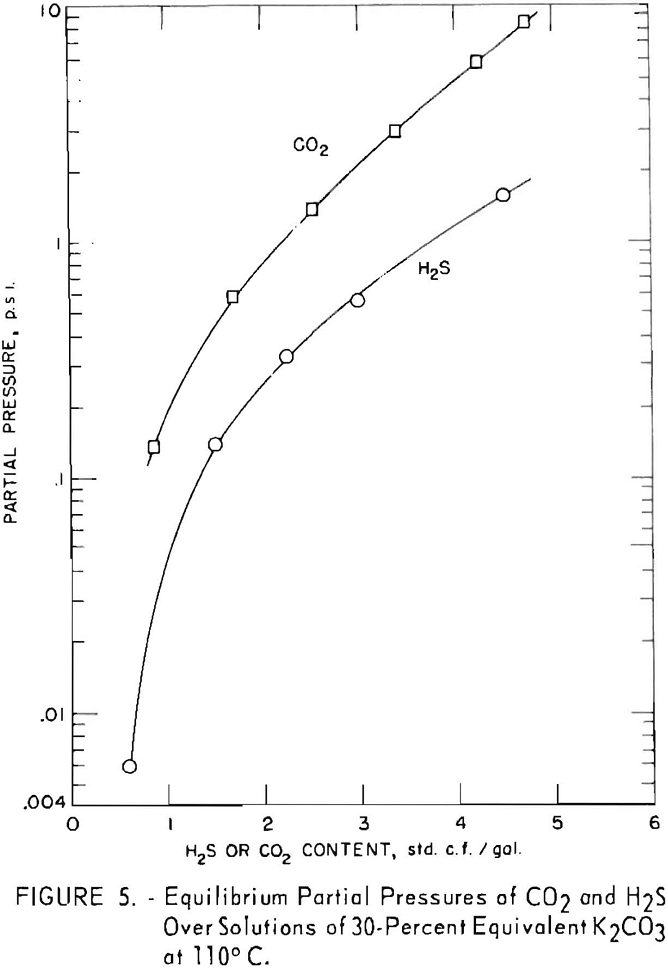
carbon dioxide is greater than that of hydrogen sulfide. To illustrate: The equilibrium pressure of carbon dioxide is 0.85 p.s.i. above the solution containing 2 cu.ft. carbon dioxide per gallon and no hydrogen sulfide, while the equilibrium pressure of hydrogen sulfide is 0.25 p.s.i. for the solution containing 2 cu.ft. hydrogen sulfide per gallon and no conversion due to carbon dioxide. The difference increases as temperature increases, since the carbon dioxide equilibrium pressure rises more rapidly with temperature than does that of hydrogen sulfide. As shown previously, there is a partial pressure of carbon dioxide as well as hydrogen sulfide above the solutions converted only by hydrogen sulfide.
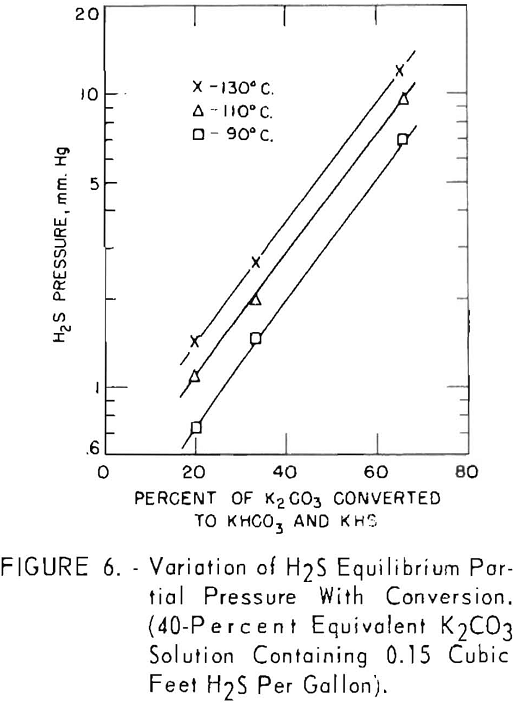
Figure 6 shows the effect of converting the carbonate solution on the equilibrium pressure of hydrogen sulfide. A 40-pct. equivalent solution of potassium carbonate containing 0.15 cu.ft. of hydrogen sulfide per gallon (100 grains per gallon) was used in these tests. Conversion of the solution was varied by adjusting the quantities of bicarbonate and carbonate charged to the autoclave. At this relatively low concentration of hydrogen sulfide, the logarithm of the equilibrium pressure of hydrogen sulfide increased linearly with conversion at the three temperature levels used.
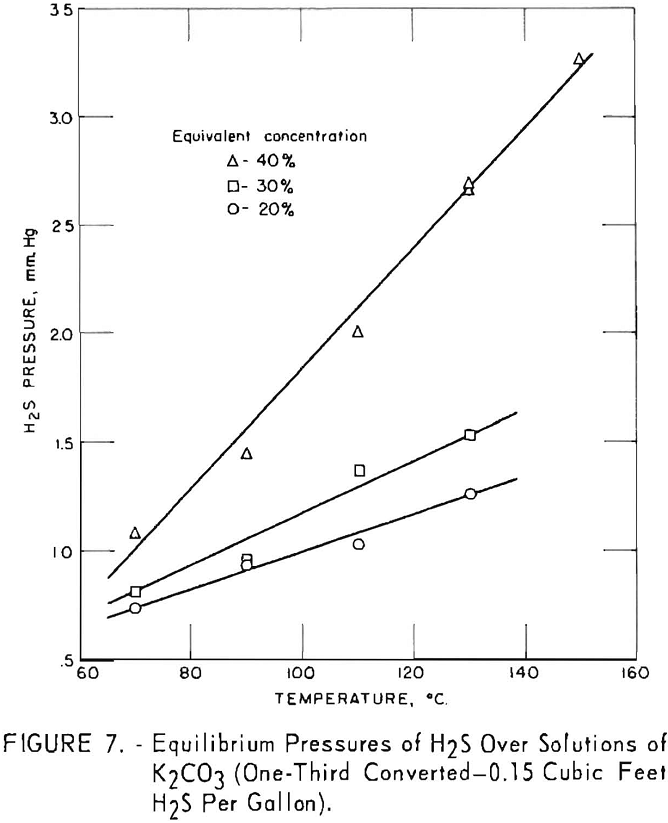
Figure 7 shows variation of equilibrium pressure with solution concentration for one-third converted solutions containing 0.15 cu.ft. hydrogen sulfide per gallon. Concentrations of 20, 30, and 40 pct. were used at temperatures of 70° to 150° C. The increase in equilibrium pressure between 20- and 30- pct. concentrations was less than between 30 and 40 pct. The difference in equilibrium pressures was greater at higher temperatures.
Solubility of Gases in Hot Carbonate Solution
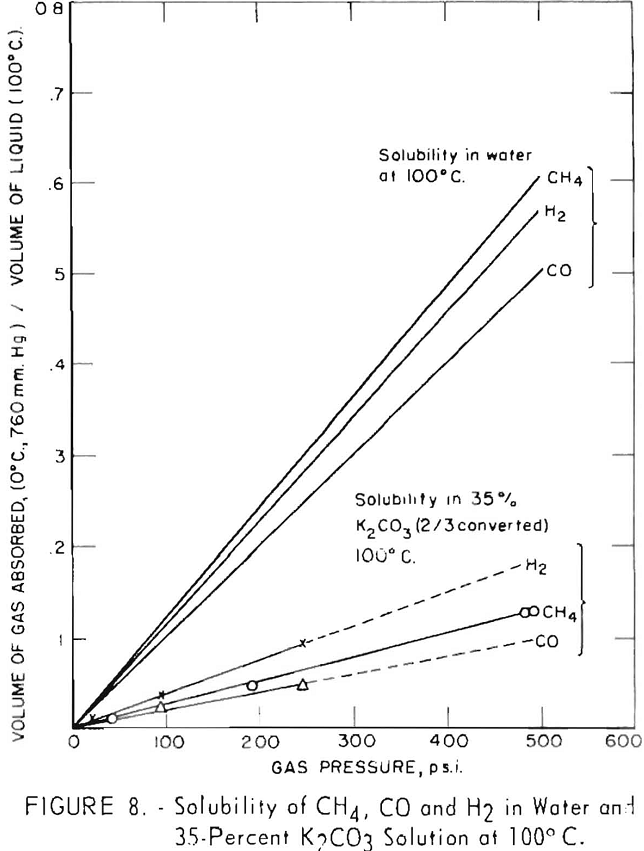
Solubilities of hydrogen, carbon monoxide, and methane were determined at 100° C. in a 35-pct. solution of potassium carbonate, two-thirds converted to bicarbonate. This is a typical conversion for spent solution leaving the bottom of the absorber in contact with the feed gas.
The gas being tested was fed into the autoclave to the desired pressure. This pressure was maintained at constant temperature for several hours while the autoclave was rocking. Hot solution was throttled into an insulated receiver and the released gases were measured and collected for analysis.
Results of these tests are plotted in figure 8, in which the volume of gas released per volume of hot solution is shown as a function of gas pressure. Solubilities of the gases are quite low; hydrogen is the most soluble and carbon monoxide is the least soluble. At gas pressures of 250 p.s.i., about 0.1 volume of hydrogen is dissolved per volume of solution, 0.07 volume of methane, and 0.05 volume of carbon monoxide. Calculated values of solubilities of the same gases in water at 100° C. are shown for comparison. Methane and carbon monoxide are about one-fifth as soluble in the 35-percent carbonate solution as in water; hydrogen is about one-third as soluble as in water.
Corrosion
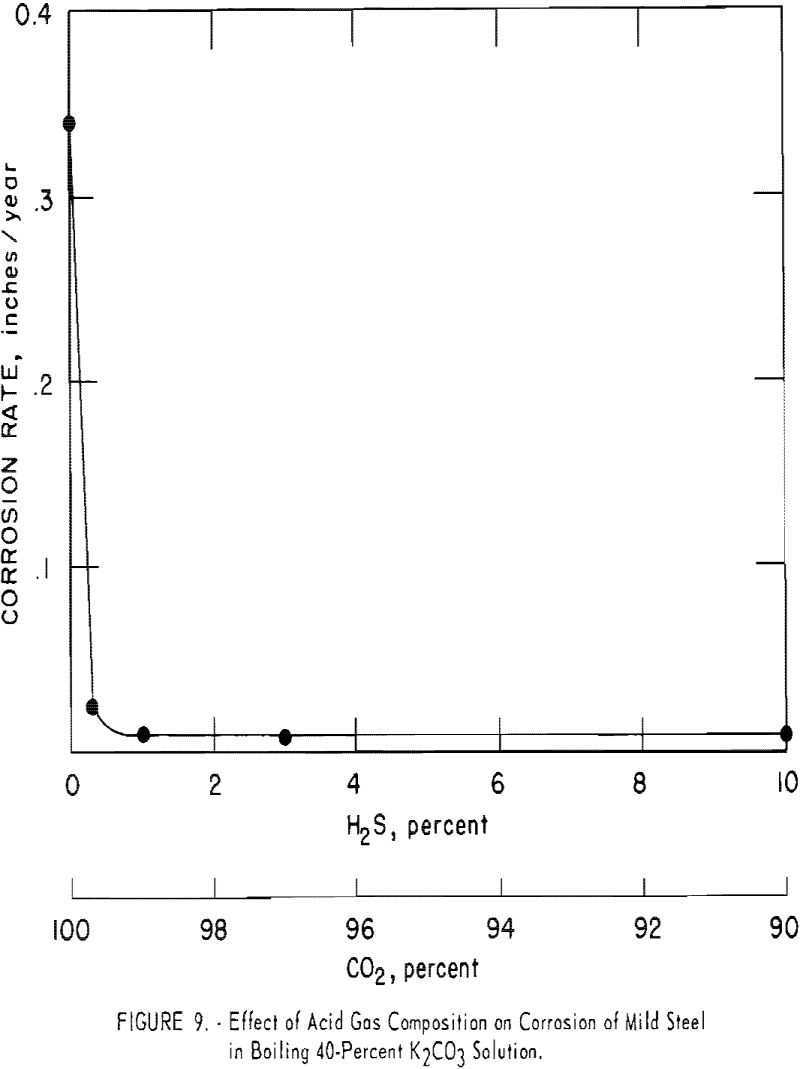
Solutions of potassium carbonate at boiling conditions are slightly corrosive to carbon steel; however, addition of carbon dioxide makes the solution extremely corrosive (0.3 in. per year) unless an inhibitor is used. Hydrogen sulfide inhibits corrosion, even in the presence of carbon dioxide. The corrosion rate diminished to about 0.008 to 0.01 in. per year at hydrogen sulfide/carbon dioxide ratios of 1:10 to 1:100. Corrosion rates were determined by bubbling the mixtures of gases through boiling 40-pct. potassium carbonate solutions that contained test coupons of carbon steel. Figure 9 and table 1 indicate the results.
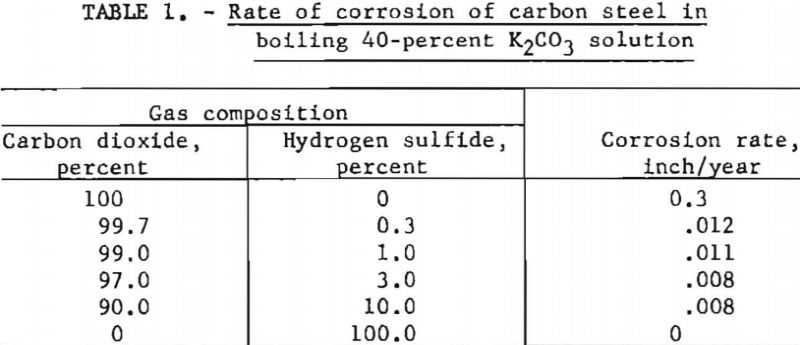
Even a small quantity of hydrogen sulfide (H2S/CO2 ratio of 1/332) reduced the attack appreciably. With hydrogen sulfide alone, there was no corrosion. Carbon steel coupons exposed to gases containing hydrogen sulfide developed a dark blue surface that remained after cleaning with steel wool. It is probable that a thin coating of iron sulfide was formed that retarded the attack.
Corrosion rates have been further reduced in laboratory tests in the presence of 99.7 pct. carbon dioxide-0.3 pct. hydrogen sulfide mixture by adding 100 to 200 parts per million of film-forming fatty amines.
Pilot-Plant Operation
Equipment
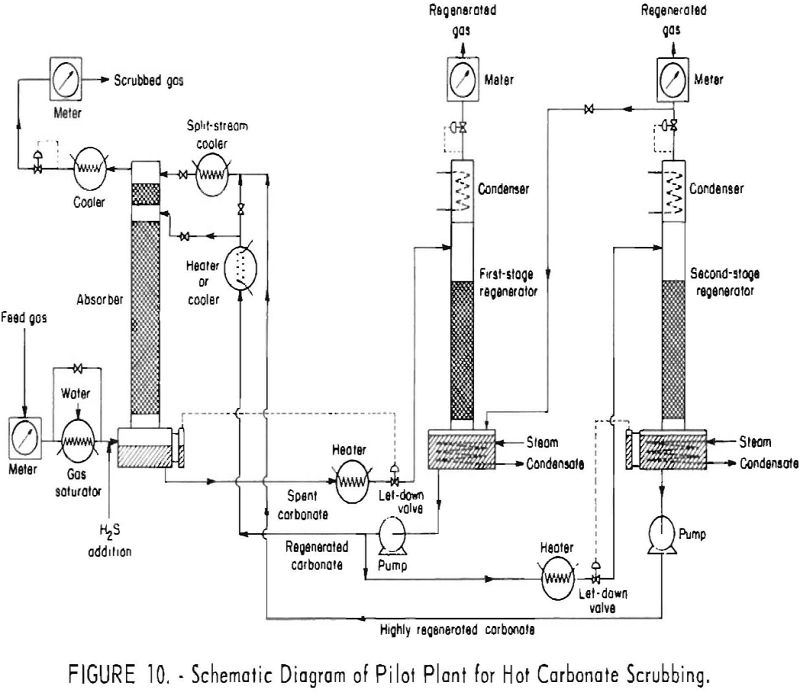
Figure 10 is a schematic diagram of the pilot plant. The valve and piping arrangement permitted the use of either single-stage, single-stage-split, or two-stage-split stream operation. The absorber was constructed from 6-in.-diameter, schedule-80 pipe, and the regenerator from 8-in., schedule-40 pipe. An additional, smaller regenerator, consisting of a 10-foot packed section of 6-in. pipe, was used to regenerate
the split stream in two-stage operation. Towers, piping, and the reboiler were carbon steel. The solution pumps, letdown valve, regenerator-condenser tubes, and some parts of the instruments were type-304 stainless steel.
The absorber contained two sections of packing: The lower section was packed to a height of 24 ft., 8 in., with ½-in. porcelain Raschig rings; the upper section originally was packed to a height of 3 ft., 10 in., with ¼-in. porcelain Raschig rings. Beginning with experiment 133, researchers increased the length of the top section of packing to 10 ft. to give a closer approach to equilibrium. The split stream entered above the top section of packing; the main stream (total stream in single-stream flow) entered above the lower section of packing.
The main regenerator contained 25 ft. of ½-in. Raschig rings. When two-stage operation was used, the total stream was withdrawn from the main regenerator and divided into two parts; the split stream was pumped to the small regenerator for further steam stripping; the main stream was pumped to the absorber entering above the lower section of packing. Acid gas liberated in the split stream was fed to the boiling solution in the main regenerator.
Feed gas was chiefly a mixture of nitrogen and carbon dioxide with small quantities of hydrogen and carbon monoxide- obtained by burning natural gas with air. The concentration of oxygen was kept low (0.02 pct. or less by mass spectrometric analysis) by limiting the air for combustion. The concentration of carbon dioxide in the gas feed to the pilot plant was varied as desired between zero and about 20 pct. by removing or adding carbon dioxide. Hydrogen sulfide was added from supply cylinders; concentrations of hydrogen sulfide were varied from less than 0.1 to 5.0 pct. with no carbon dioxide present, and from less than 0.1 to 12.0 pct. in tests with carbon dioxide in the gas. Carbonyl sulfide also was fed into the gas stream from supply cylinders.
Pilot-Plant Results
Typical Data With Various Feed Gases
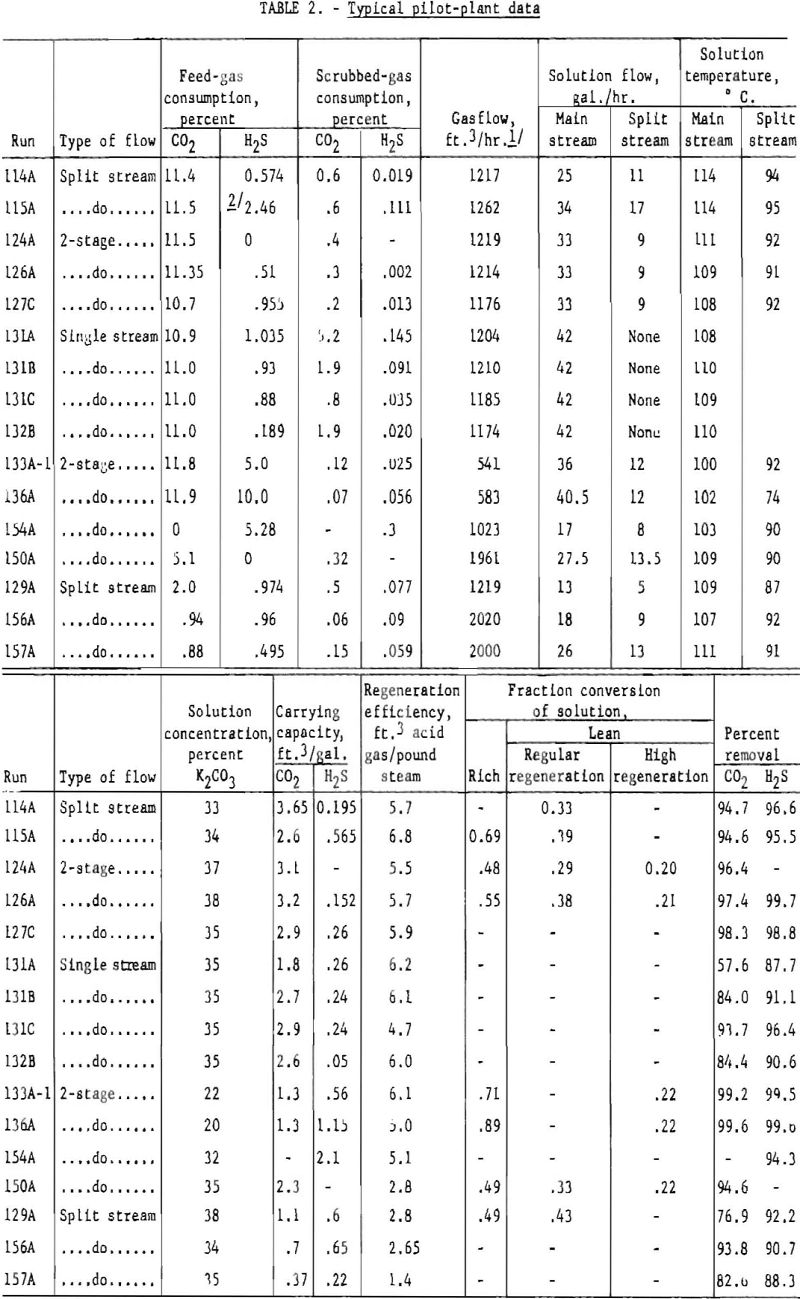
About 100 tests with gases containing hydrogen sulfide were made in the pilot plant. Table 2 shows typical data with various types of flow and different percentages of hydrogen sulfide and carbon dioxide in the feed gas. The absorption pressure was 300 p.s.i.g., and the regeneration pressure slightly above atmospheric in all tests. No attempt was made to remove the hydrogen sulfide selectively. The degree of carbon dioxide removal was chosen, and the accompanying removal of hydrogen sulfide was determined.
Degree of Removal of Carbon Dioxide and Hydrogen Sulfide
Several tests used feed gases containing about 0.5 and 1.0 pct. hydrogen sulfide and 1 to 20 pct. carbon dioxide to determine the variation of hydrogen sulfide removal with carbon dioxide removal. These concentrations of hydrogen sulfide and carbon dioxide cover the range obtained In synthesis gas produced by gasifying coal. Solution-carrying capacities and regeneration efficiencies were about the same as one might expect if the total acid gas were carbon dioxide.
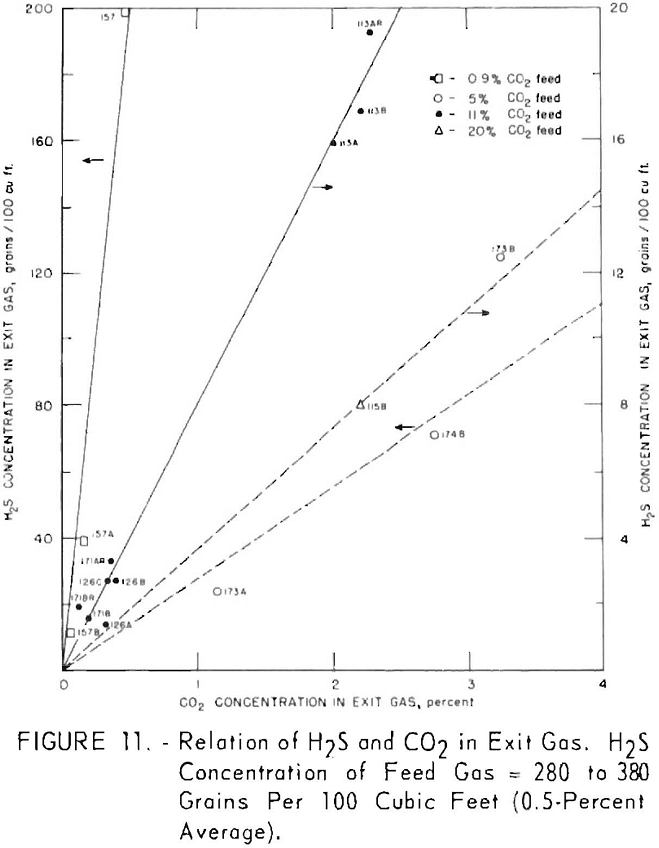
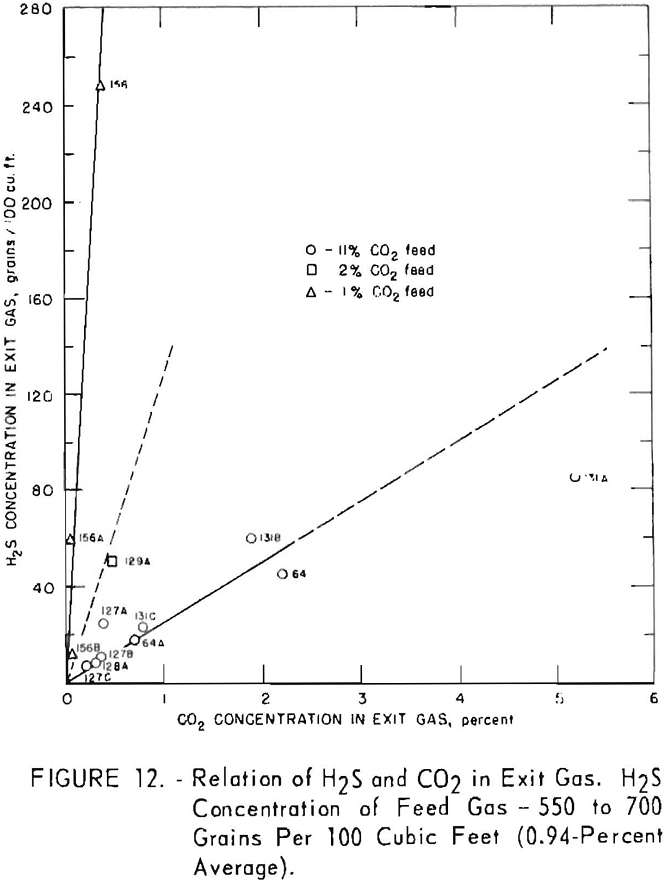
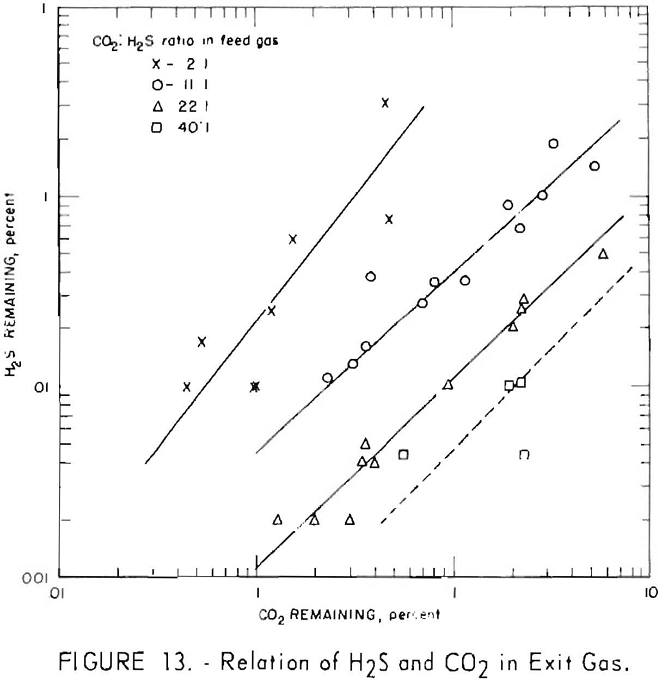
Figures 11 and 12 illustrate variation of residual hydrogen sulfide with residual carbon dioxide in the purified gas for different concentrations of carbon dioxide in the feed gas. Hydrogen sulfide concentrations in the feed gas were 280 to 380 grains per 100 cu. ft. (approximately 0.5 pct. average) in the tests plotted in figure 11, and 550 to 700 grains (approx, 1 pct. average) in the tests plotted in figure 12. Figure 13 shows a more generalized relationship between the removal of hydrogen sulfide and that of carbon dioxide over a wide range of feed gas concentrations. The hydrogen sulfide concentration in the feed gases varied from 0.2 to 5.0 pct. in the tests shown in figure 13. Both single- and split-stream flows are represented, the split-stream flow for obtaining lower residual concentrations of carbon dioxide and
hydrogen sulfide. Gas and liquid flows and temperatures favorable to carbon dioxide removal were chosen, and no attempt was made to operate at conditions for selective removal of hydrogen sulfide.
At each concentration of carbon dioxide in the feed gas, the residual concentration of hydrogen sulfide decreased proportionally to the residual concentration of carbon dioxide. Also, for a given concentration of carbon dioxide in the purified gas, the residual concentration of hydrogen sulfide decreased as concentration of carbon dioxide in the feed gas increased. The more thorough regeneration of the lean solution required for increased removal of carbon dioxide resulted in decreased equilibrium pressure of hydrogen sulfide above the solution, thereby removing more hydrogen sulfide. Doubling the concentration of hydrogen sulfide in the feed resulted in an approximate twofold to threefold increase in the residual concentration of hydrogen sulfide for the same carbon dioxide concentrations.
Concentration of Hydrogen Sulfide and Carbon Dioxide Along the Absorber
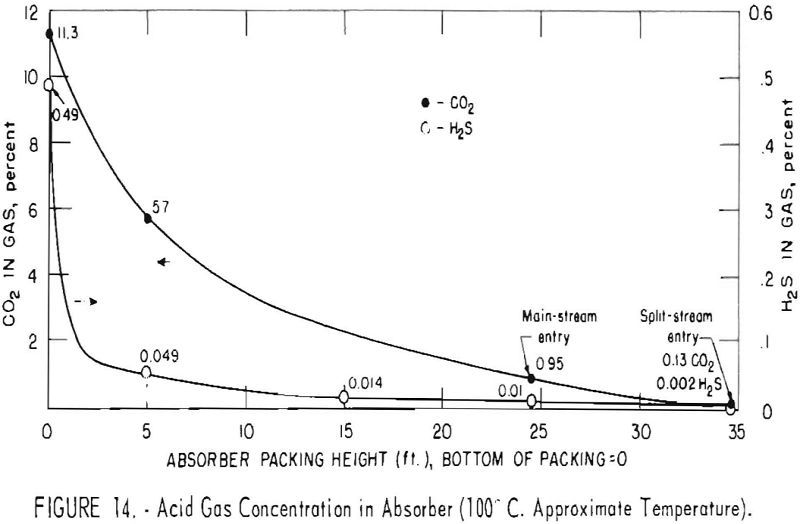
Concentrations of carbon dioxide and hydrogen sulfide were measured along the absorber to observe the relative rates of removal of the two gases in test 171B-r. These results are plotted in figure 14. The feed gas contained 11.3 pct. carbon dioxide and 0.49 pct. hydrogen sulfide, and the outlet gas contained 0.13 and 0.002 pct. of these components, respectively. Split-stream flow with two-stage regeneration was used with a solution of 35-pct. concentration. The fraction conversion to bicarbonate of the cooled split stream entering at the top of the column was about 0.20; that of the main stream entering at the 25-ft. level was 0.36. Concentrations of hydrogen sulfide in the split and main streams were about 0.20 and 0.26 cu. ft. per gallon.
As shown in figure 14, the absorption of hydrogen sulfide was rapid. Concentration declined from the initial value of 0.49 to 0.049 pct. by the time the gas reached the first sampling point at the 5-ft. level. Between the same points, the concentration of carbon dioxide was lowered from 11.3 to 5.7 pct. About 53 pct. of the carbon dioxide and slightly more than 90 pct. of the hydrogen sulfide had been removed. This Indicates that a high degree of selectivity can be achieved with a short time of contact.
At the next sampling level, 15 ft. from the bottom in this test, the hydrogen sulfide was 0.014 pct., and its concentration thereafter remained essentially constant in the main stream section of the absorber (to the 25-ft. level). No measurement of carbon dioxide was made at the 15-ft. level, but at the top of the main section it was 0.95 pct. The cooled and more highly regenerated split stream finally reduced the concentration of hydrogen sulfide to 0.002 pct., and that of the carbon dioxide to 0.13 pct. in the top 10-ft. section of packing.
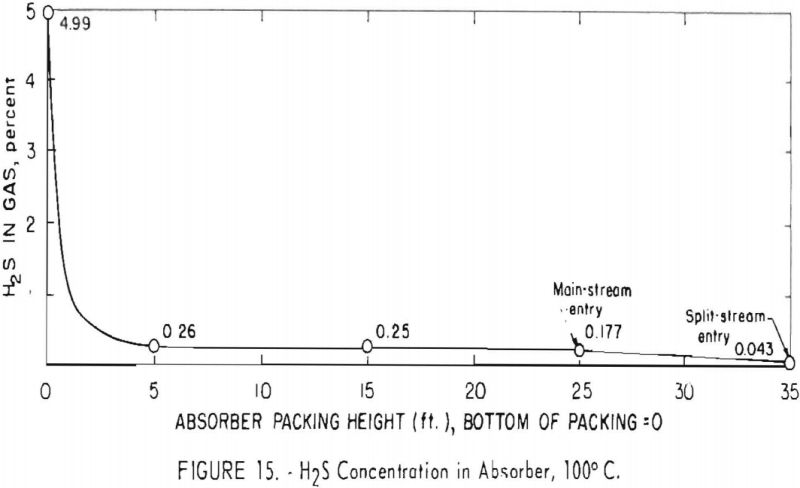
Figure 15 depicts a profile of the concentration of hydrogen sulfide in the absorber for test 172, in which the feed gas contained about 5 pct. hydrogen sulfide and was free of carbon dioxide. A solution of 35-pct. concentration was used, and the solution-carrying capacity was 2.7 cu.ft./gal. The high rate of absorption of hydrogen sulfide is shown by the removal of 95 pct. in the bottom 5 ft. of packing; concentration dropped from 4.99 to 0.26 pct. in this section. Equilibrium between the gas and liquid is indicated because the concentration of hydrogen sulfide was not lowered further until the 25-ft. level at the top of the main section. Here the composition and temperature of the carbonate solution were altered by the addition of the split stream cooled to 50° C.
Removal of Carbonyl Sulfide
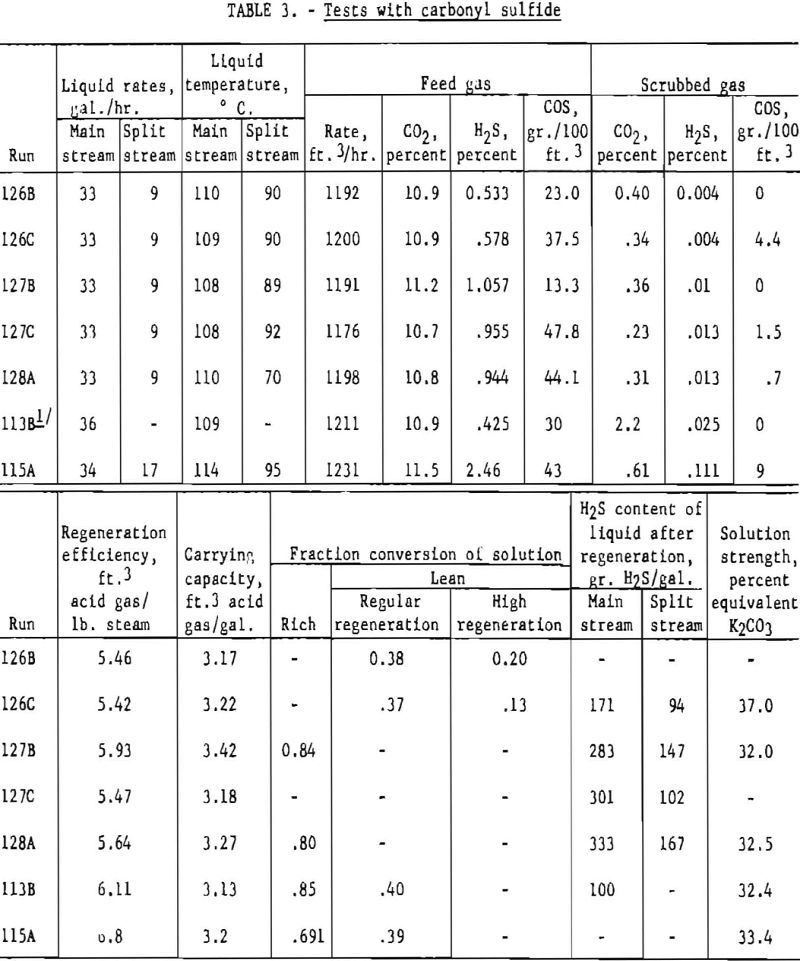
Table 3 shows results of tests made with gases containing carbonyl sulfide, hydrogen sulfide, and carbon dioxide. Split-stream flow with two-stage regeneration was employed in all tests except 113B, for which a single stream was used. Carbonyl sulfide is hydrolyzed by hot carbonate solution, and in several tests complete removal was accomplished. Some unreacted carbonyl sulfide was found in the purified gases when feed gases contained about 40 grains of carbonyl sulfide per 100 cu.ft. This was especially characteristic of test 115A, in which the feed gas contained a relatively high concentration of hydrogen sulfide. Results of the bench-scale tests given earlier indicate that a high absorption temperature favors the removal of carbonyl sulfide.
Selective or Final Hydrogen Sulfide Removal
To attain greater hydrogen sulfide removal, experimenters made a few tests with the split stream or with the entire stream at temperatures lower than ordinarily used in hot carbonate operations. Bench-scale tests on relative absorption rates showed that hydrogen sulfide was removed considerably faster than carbon dioxide. The rapid removal of hydrogen sulfide was confirmed in the pilot plant by the measurements of hydrogen sulfide concentrations along the absorber.
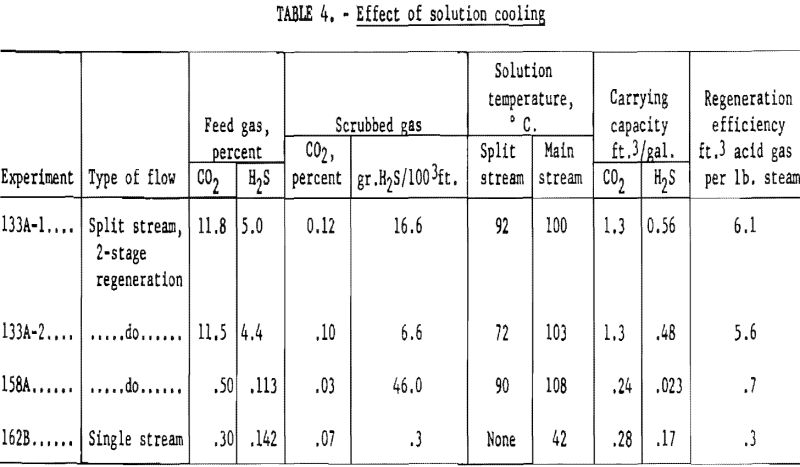
Table 4 contains data on the effect of lower solution temperature on the removal of hydrogen sulfide. Tests 133A-1 and 133A-2 used a feed gas containing about 11.5 pct. carbon dioxide and 5 pct. hydrogen sulfide. The gas and liquid rates were the same, but the split stream temperature was 92° C. in 133A-1, and 72° C. in 133A-2. The final concentration of hydrogen sulfide was 16.6 grains in 133A-1 and only 6.6 grains in 133A-2 with the cooler split stream.
In tests 158A and 162B the advantage of using a relatively cold stream of potassium carbonate for final removal of hydrogen sulfide is illustrated. The feed gas contained 0.3 to 0.5 pct. carbon dioxide and 0.11 to 0.15 pct. hydrogen sulfide. This concentration of hydrogen sulfide is higher than would be expected from an earlier hot carbonate purification at the usual temperatures but was used for ease of preparation and analysis. Two-stage regeneration was used in test 158A, with the main stream entering the absorber at 108° C. and the smaller stream at 90° C. The purified gas contained 46 grains of hydrogen sulfide per 100 cubic feet (0.069 percent) and 0.03 percent carbon dioxide. A single stream in 162B at a temperature of 42° C. gave a purified gas containing 0.3 grain hydrogen sulfide per 100 cu.ft., and 0.07 pct. carbon dioxide.
An added margin to insure low concentration of hydrogen sulfide is the use of a few trays in the top section of the final absorber, to which the makeup solution (either KOH or K2CO3) is added. Losses of solution from leakage through pump packing or any other cause could be replaced in this manner. This was demonstrated in bench-scale equipment, in which a bleed stream of purified gas from the hot carbonate absorber was bubbled through a ½-in, level of 25-pct. potassium carbonate solution (135 cc. of solution) at 300 p.s.i.g. pressure and room temperature to determine the removal of hydrogen sulfide and carbon dioxide. Results were as follows:
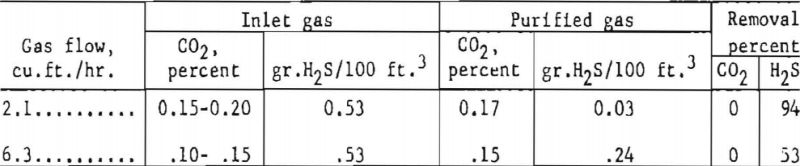
Carbon dioxide did not react because the rate of reaction was too slow. If potassium hydroxide were used instead of the carbonate, the concentrations of both carbon dioxide and hydrogen sulfide would be lowered. However, the potassium hydroxide would be converted rapidly to potassium carbonate by the carbon dioxide, and the selectivity of the carbonate solution would be as demonstrated earlier.
