Table of Contents
An ion exchange resin was found to quantitatively and selectively collect gold from acid solutions over a wide range of acid concentrations. Common metals at high concentration are not collected by the resin and they do not seriously interfere with the collection of gold. Alkaline cyanide solutions gradually destroy the capability of the resin to collect gold, but do not cause the release of the gold initially collected.
Ion exchange resin-loaded papers containing 50 percent resin are an excel¬lent medium for collecting gold in a form suitable for X-ray spectrographic and neutron activation determination. In both analytical methods, cyclotron- produced 195Au can be used as a collection monitor.
A paper disclosed information on the separation of noble metals from base metals by means of a new chelating resin described as a styrene-divinyl-benzene copolymer into which a resonating amino group has been introduced. They reported the quantitative collection of Os+8, Rh+³, Ir+4, Pd+², Pt+4, and Au+³ and gave data to show that the presence of Ni+², Cu+², and Fe+³ had little or no adverse effect on the collection of Pt+4. Their statement that 1 gram of the dry resin had a capacity for 1.1 grams of gold was of particular interest.
Because of its potential importance in the Bureau of Mines Heavy Metals Program, additional tests were performed to obtain more detailed information on the use of this resin in analytical operations.
Experimental Procedure
The resin (minus 16 plus 50 mesh) was used as purchased. Solutions of known gold concentration were prepared from high-purity gold foil. All reagents and inorganic salts of the common metals were reagent grade. Gold radiotracer solutions were prepared from carrier-free 195Au with a radiochemical purity of greater than 99 percent.
Ion exchange column tests were performed in a glass chromatographic tube with a 1-cm ID. The resin was supported in the column by means of a fritted glass plate. A glass delivery tube was connected to the lower end of a chromatographic tube by means of a short piece of rubber tubing. A screw clamp on this rubber tubing was used to control the flow rate from the column at approximately 2 ml/min. A 5-cm resin column was used in the initial acid studies; for other tests a 3-cm column of resin was used. A separatory funnel mounted above the chromatographic column served as a reservoir for the test solution and was used to maintain the test solution at a constant level in the chromatographic tube until all the test solution had passed through the resin. All test solutions contained sufficient 195Au to provide good counting rates. and. except where otherwise noted. contained 20 µg/ml of inactive gold. A few drops of concentrated nitric acid were added to the 195Au tracer solution and to the solutions of common metals to prevent reduction of gold by trace impurities in the chloride salts from which these metal solutions were prepared. The test solutions were diluted to 250 ml before they were passed through the ion exchange column.
An aliquot of the original test solution and an aliquot, or aliquots, of the column effluent were taken for counting of gamma activity. Counting was done by means of a well-type NaI (T1) detector and single channel analyzer. The percentage of gold retained by the resin was calculated from the gamma activities of the initial test solution and the effluent:
Au retained, percent = 100 (1 – A2/A1)
where A1 = activity of the initial test solution
and A2 = activity of the effluent.
Tests involving the use of ion exchange resin-loaded paper disks were performed using the filtering apparatus and technique described by Campbell, Spano, and Green. The resin-loaded disks used in these tests were prepared for the authors by the H. Reeve Angel Co.
Results
Effect of HCl and HNO3
In analytical methods, aqua regia is the preferred reagent for dissolving gold. Therefore, initial tests were performed to determine the effect of HCl, HNO3, and mixtures of HCl and HNO3 on the retention of gold by the resin. The results of these tests are given in table I.
At the highest concentration of mixed HCl and HNO3, a breakthrough was observed (fig. 1) after approximately 30 minutes exposure of the resin to the mixed acid. Prior to this breakthrough, small aliquots of the effluent showed
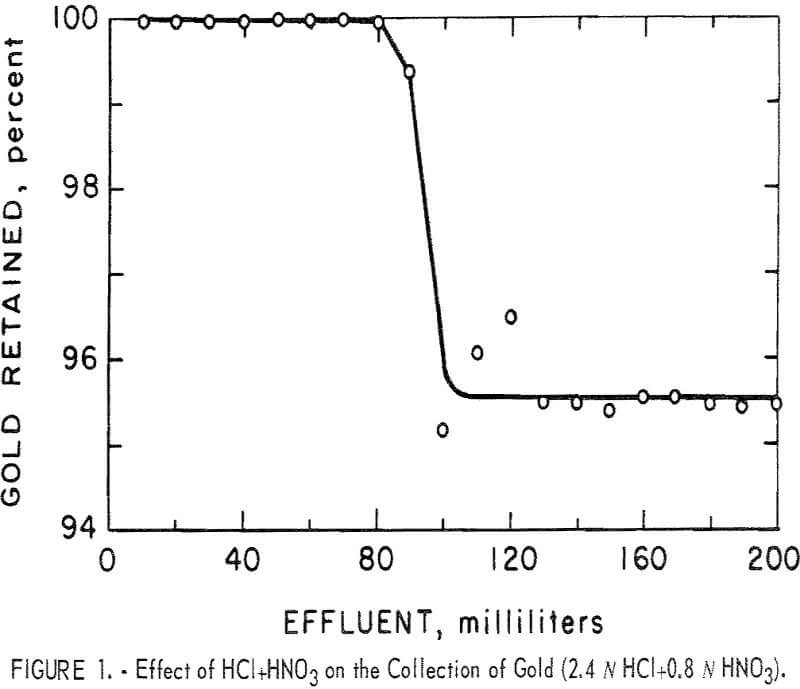
that essentially all the gold was collected from the solution. After the breakthrough gold retention dropped to between 95 and 96 percent and remained at that level until the end of the test. During this test, darkening of the resin was observed. By the time the breakthrough point was reached, the entire column of resin had turned dark brown.
At higher concentrations of aqua regia, the resin is slowly attacked and its ability to quantitatively collect gold is destroyed. The following test was performed to determine whether the gold initially collected by the resin was retained after prolonged treatment with 50 percent aqua regia (3 volumes HCl, 1 volume HNO3, 4 volumes H2O). A 1-gram portion of the resin was continuously stirred in a 50-percent aqua regia solution containing 20 mg of inactive gold and 195Au tracer. The percentage of gold remaining in the solution was periodically determined. The results given in table 2 show that the collection of gold is slow and not quantitative, but that the gold which has been collected is retained by the resin. In practical applications it would not be necessary to work at such high acid concentrations.
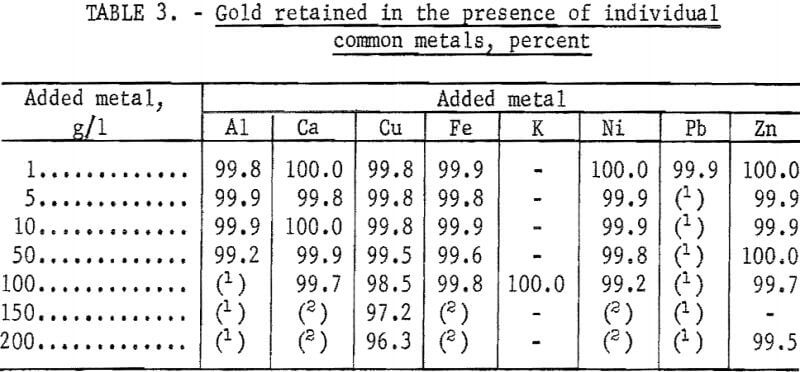
Effect of Common Metals
Koster and Schmuckler reported that nickel, cobalt, and iron had little effect on the collection of platinum by the resin. They did not report the effect of common metals on the collection of gold. In our tests, 100-ml portions of solutions containing 20 mg/l of gold and a wide range of concentration of the common metals were adjusted to 1.2 N with HCl and passed through 3-cm-long columns of the resin at the rate of 3 ml/min. The results are given in table 3.
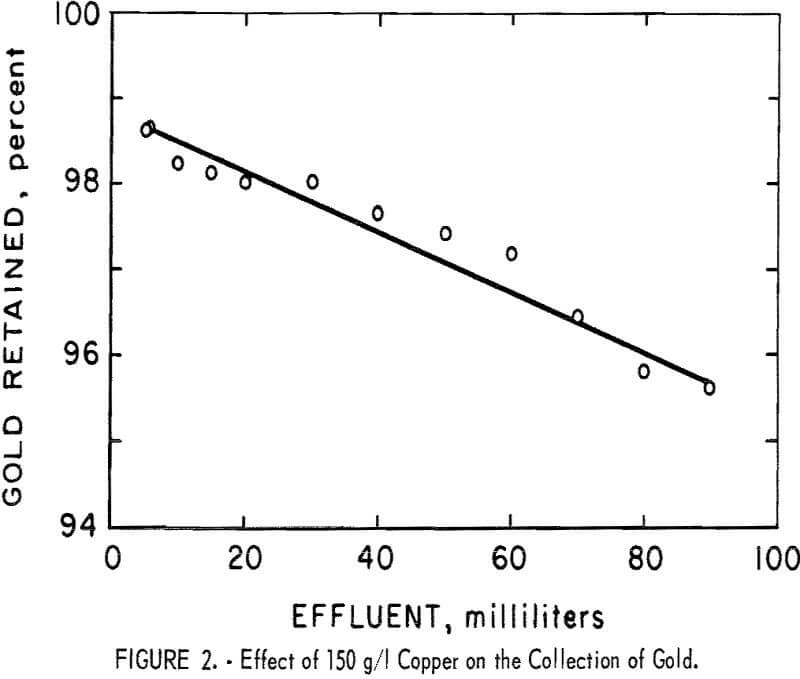
Retention of gold by the resin was essentially quantitative in the presence of all the common metals except copper. Because the effect of copper was different, the test in the presence of 150 g/l of copper was repeated under the same conditions except that the gold was determined in small consecutive fractions of the eluate. The results of this test, shown in figure 2, indicate that the quantity of gold retained gradually decreased and that no sharp breakthrough occurred.
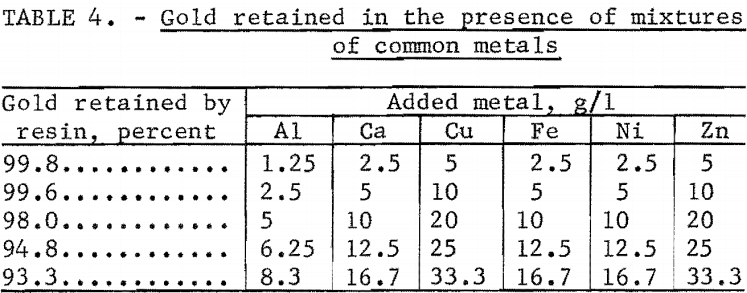
The effect of combinations of common metals on the retention of gold was determined using the same experimental technique. The results given in table 4 indicate that the combined effect of these metals is greater than the effect of any single metal. It should be noted that these interelament effects were studied at concentrations that greatly exceed those anticipated in practical applications. If such high concentrations were encountered, simple dilution would restore the ability of the resin to quantitatively collect gold.
Use in Alkaline Cyanide Solutions
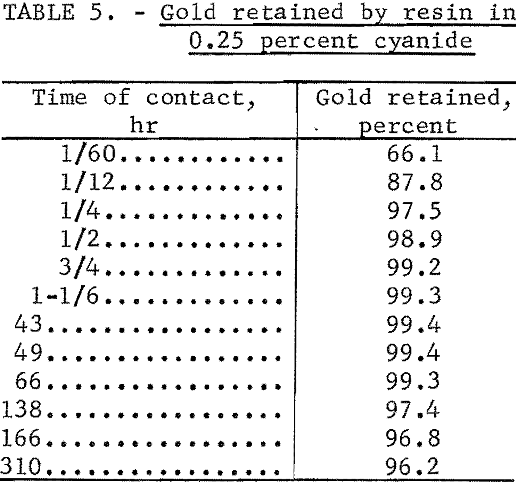
Koster and Schmuckler stated, “The noble metals must be adsorbed on the resin in an acidic medium, as in an alkaline environment, hydrolysis of the metals as well as of the amine groups of the resin takes place,” Tests which we performed showed that cyanide solutions containing either NaOH or CaO as the alkaline constituent slowly attack the resin. However, during initial contact between 0.25 percent cyanide solution and the res in, gold is nearly quantitatively collected by the resin. The gold which has been collected is retained by the resin after the capability of the resin to collect gold has been destroyed.
Table 5 shows the results of a test in which a 1-gram portion of the resin was added to a flask containing 50 ml of 0.25 percent NaCN solution in which 1,000 µg of gold and 195Au tracer were dissolved. The flask was shaken periodically, portions of the solution were temporarily removed for counting, and comparison was made with the radioactivity of an identical solution to which no resin had been added.
An ion exchange column test was performed using an alkaline 0.25 percent solution of NaCN containing 20 mg/l of gold and 195Au radiotracer. The flow rate through the 10- by 30-mm column was adjusted to 2 ml/min. The gold
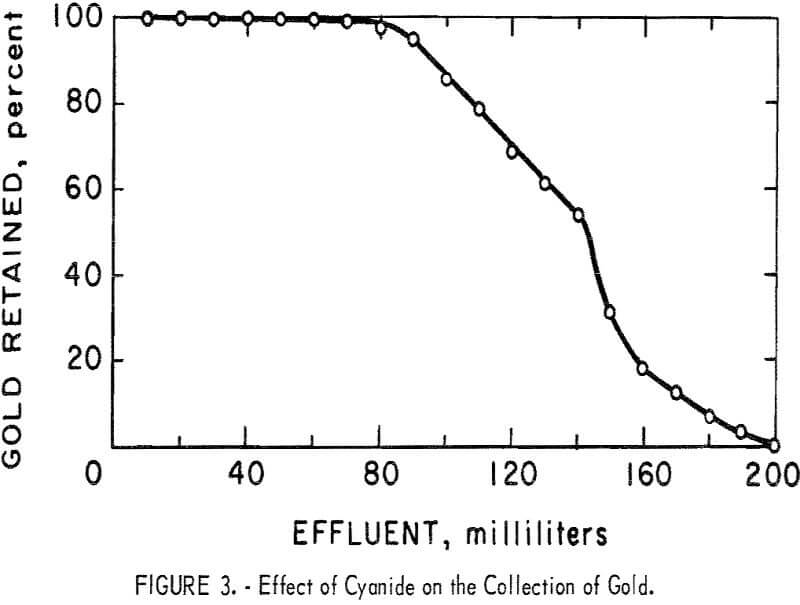
content of successive small fractions of the effluent were determined by gamma counting. The results are shown in figure 3.
During the passage of the first 100 ml of solution through the column, a yellow discoloration of the effluent was observed indicating a reaction between the resin and the alkaline solution. After the passage of the first 100 ml, the yellow discoloration was no longer observed. Even though the resin rapidly lost its ability to retain gold at this point, no tendency was observed for the resin to lose the gold it had already collected.
Ion Exchange Resin-Loaded Papers for Analytical Use
Thin disks, 3.5 cm in diameter, consisting of 50 percent cellulose and 50 percent resin were used to explore the possibility of collecting gold for determination by X-ray spectrography or neutron activation. Previous work with similar disks containing nonselective ion exchange resin had shown that such disks provided an excellent medium for collecting trace metals for X-ray spectrographic determination. The efficiency of resin-loaded disks for collecting gold was evaluated by filtering 50-ml portions of 5 percent HCl solutions containing 500 µg of 197Au and tracer quantities of 195Au. Gamma counting of the filtrates showed that one filtration resulted in the collection of 93 percent of the gold; five to seven passes of the solution through the paper resulted in collection of 99.5 percent of the gold. A similar test using 0.25 percent NaCN solutions resulted in less than 10 percent of the gold being collected. For analytical purposes cyanide solutions could be converted to chloride solutions from which the gold is quantitatively collected. X-ray spectrographic sensitivity was determined on 3.5-cm disks of this paper on which 500 µg of gold had been collected. Using a tungsten target tube at 50 kv and 40 ma, a sensitivity of 2.2 counts per second per microgram above a background of 300 counts per second was obtained. Fifty micrograms of gold on a 1.0-cm-diam disk produced a sensitivity of 6.5 counts per second per micro-gram above a background of 140 counts per second.
In previous ion exchange column tests, the resin had been shown to be highly selective for gold in the presence of large quantities of common metals. Additional tests were performed to determine the effect of common metals on the collection of gold by the ion exchange resin-loaded paper disks. The experimental conditions and results of these tests are given in table 6. The percentage of gold retained on the disks was determined by measuring the activity of the 196Au in the filtrate. Other tests were performed under the same conditions as in test 3 of table 6, except that the volume of the solution was increased. Results are shown in table 7.
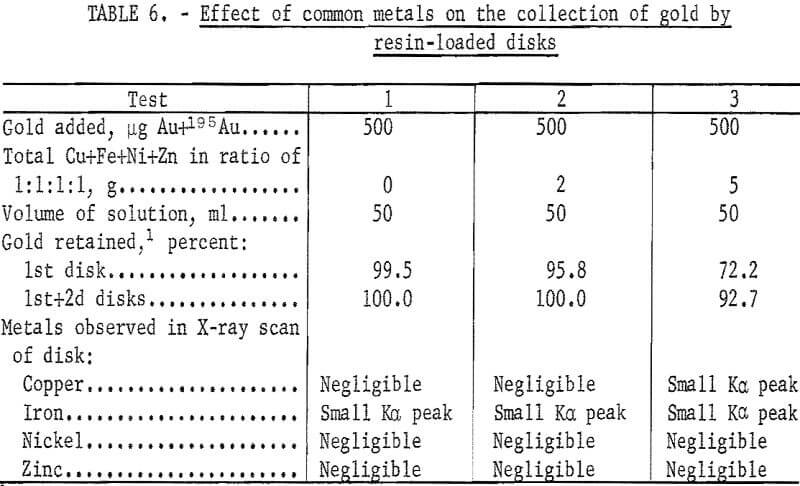
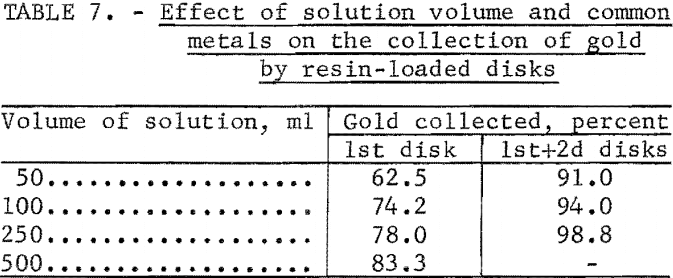
Although increasing the volume of the solution reduced the interference of the common metals, the filtering rate of 10 ml/min places a limit on the volume which can be conveniently used.
These results show the advantage of using 195Au as a collection monitor. For analytical purposes, the collection of gold need not be complete as long as the percentage of collection is known. The small amounts of the common metals collected caused no interference in the measurement of the Lβ1 X-ray peak of gold.
Greater sensitivity can be obtained if the gold collected on the disks is determined by neutron activation. During activation, the naturally occurring 197Au from the sample is converted to 198Au with a gamma peak at 0.412 mev. The 195Au, which has a gamma peak at 0.130 mev, is essentially unaffected by the neutron flux. Gamma counting over these two energy ranges provides a means to determine the gold content of the original sample. Standard disks containing known quantities of 197Au and 195Au, and test disks containing 197Au collected from weighed quantities of Bureau of Mines reference ore plus known quantities of 195Au were sent to Gulf General Atomic for neutron activation and gamma counting. A linear relationship between gamma intensity and amount of gold added was obtained over the 0.1 µg to 1.0 µg range of these tests.
A major advantage of collecting gold in the presence of 195Au monitor prior to neutron activation is the elimination of high levels of activity which are produced if the entire sample is activated. A technical publication describing both the X-ray spectrographic and neutron activation methods is now being prepared.
