Table of Contents
The problem of treating oxidized lead-zinc ores for the production of high-grade lead zinc flotation concentrates is a complex problem due to the nature of the ores and to the soft sliming characteristics of the lead and zinc minerals. The ore for treatment is a lead-zinc carbonate ore in a mixed siliceous-lime carbonate gangue. The association of the minerals and gangue requires a 65 mesh grind for liberation. The problem is to produce marketable grades of lead and zinc float concentrates.
Pb Zn Flotation Circuit
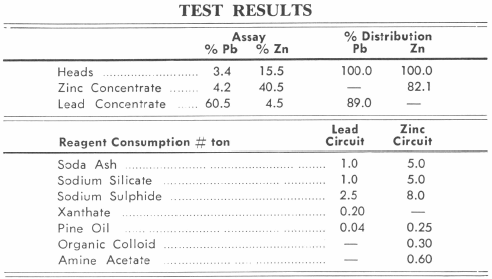
This flowsheet was developed from an exhaustive study of the minerals involved. Many theories were explored to answer the objective of producing marketable grades of lead and zinc economically. The simplicity and flexibility of this flowsheet make it particularly desirable in a period of low base metal prices. The use of a sulphidizer to activate the oxidized lead minerals and control and/or removal of reagent consuming slimes is provided ahead of the oxidized zinc flotation section.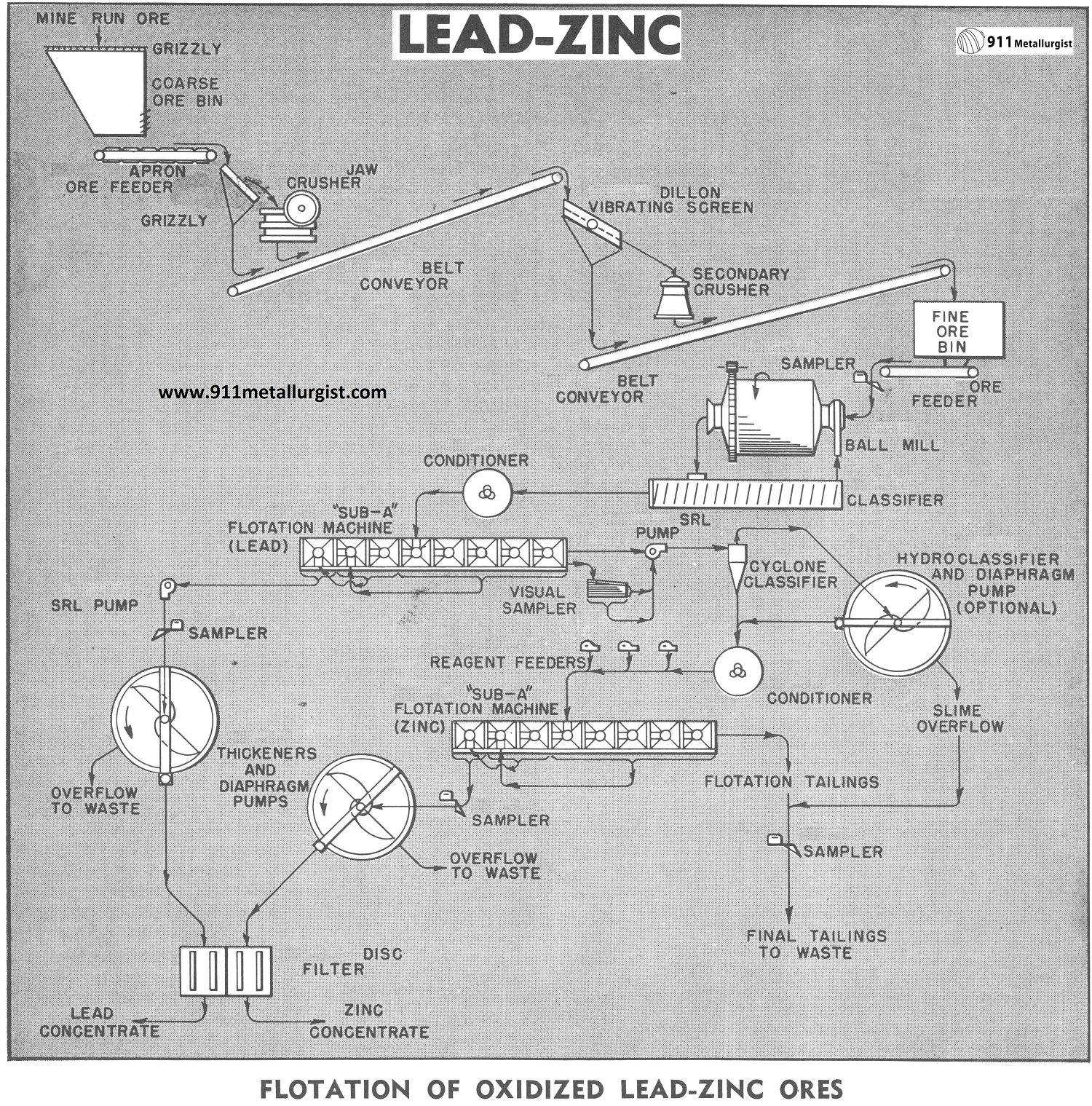
Crushing Pb Zn Ore
The flowsheet uses a two- stage open circuit crushing section with a capacity of 100-275 tons per 24 hours to reduce the ore to minus ¾ inch for feed to the ball mill. The size of crushing equipment will depend on the size of ore and tonnage of material delivered to the crusher.
GRINDING Lead Zinc Ore
The crushed ore minus ¾ inch is fed by means of a belt ore feeder to a Ball Mill which operates in closed circuit with a spiral classifier to produce a minus 65 mesh classifier overflow product.
Conditioning and Flotation of Pb and Zn
The classifier overflow passes to a Conditioner where sodium sulphide is added to sulphidize the oxidized lead minerals. The sulphidized lead minerals are subjected to flotation using xanthates or mercaptans and pine oil to produce a flotation concentrate which is cleaned two times to produce a high grade marketable lead concentrate.
The flotation tailing from the lead section passes to a wet cyclone to make a primary sand-slime separation. The cyclone overflow passes to a Hydroclassifier which is operated to overflow a turbid overflow which contains the minus 20 micron slimes. This step is often necessary to remove the fine slimes which interfere in the flotation of the oxidized zinc minerals.
The combined underflows from the Hydroclassifier and cyclone represent the feed to the zinc flotation section. The combined products flow to a zinc conditioner where soda ash, sodium silicate and an organic colloid are added to disperse the pulp and complex the slimes which might interfere with flotation. The additional reagents required for the zinc flotation consist of sodium sulphide, primary amines and a frother which are added to the conditioner discharge in the order stated and prior to entering the flotation machines. The rougher zinc concentrate is cleaned two times to produce a high grade zinc concentrate.
The lead and zinc flotation was conducted in “Sub-A” Flotation Machines which provides for the maximum grade of concentrates and the maximum flexibility for the continuous retreatment of the cleaner middlings.
Thickening and Filtering of Zn and Pb
The final lead and zinc concentrates are pumped by concentrate pumps to Thickeners for dewatering prior filtration. The thickener underflow at approximately 50% solids is pumped to a Disc Filter by means of a Diaphragm pump. The filter cakes pass to concentrate bins for storage.
Zn Pb Plant Sampling
Automatic Samplers are used to sample the feed, concentrate and tailings. A portion of the tailing from the lead flotation section is removed and treated on the Visual Sampler. The flotation results and conditions can be controlled easily by observing the concentrate streak on the table.

ORE TESTING LABORATORY
The Ore Testing Laboratory is available to help you determine the proper flowsheet for your Ore. There is no substitute for experience. Consultation is without obligation. Charges for test work represent out out-of-pocket expenses only and are recommended as a means of eliminating risk in mining investments. Additional details available on request.
Lead Zinc Flotation
Several processes have been developed for the separation of lead from zinc sulphides by two-stage selective flotation, but the only method that is employed widely enough to warrant a description in these pages is the one whereby the zinc minerals are depressed by sodium cyanide and/or zinc sulphate, allowing the galena, which remains unaffected, to be floated in the first stage. Although the primary function of the cyanide is to depress the zinc sulphides, it also has a similar action on any pyrite present. The addition of zinc sulphate generally, but not necessarily, intensifies the depressing effect; in many plants cyanide alone is used. On the other hand, in some cases better results have been obtained by the use of zinc sulphate without cyanide, but the method is not, as a rule, successful if pyrite is present, since zinc sulphate has not usually a sufficiently powerful depressing action on the iron sulphides.
The pulp is generally maintained at an alkalinity of pH value 7 .5-8.0 with soda ash, which also serves to precipitate dissolved salts released during grinding. It is common practice to put the soda ash into the primary ball mill and to add the depressing reagents at some later point in the grinding circuit. Sodium cyanide and zinc sulphate normally require a contact period with the pulp of 10 to 20 minutes, and since it is occasionally necessary and generally preferable to control this time with considerable accuracy, a conditioning tank is usually installed for the purpose ahead of the flotation machines of the first stage. The galena is then floated with the addition of cresylic acid or pine oil in conjunction with one or more of the xanthates or aerofloat, the zinc minerals passing in the tailing to the second stage of flotation.
Cresylic acid is generally preferred to pine oil on account of its greater selectivity ; should the froth be too delicate, coal-tar creosote is added to stiffen it. Ethyl and amyl xanthates, either alone or in combination, can be used as promoters, but aerofloat with or without a small amount of thio-carbanilide sometimes proves more satisfactory. With pyritic ores the latter mixture generally has the advantage that flotation of the galena is easier to control, since the reagents have less tendency to bring up pyrite than when xanthates are used. Lime may be substituted for soda ash, but, owing presumably to its depressing effect on sulphide minerals, including galena, its use is not satisfactory unless one of the more powerful higher xanthates is employed, notably amyl xanthate. The action of a higher xanthate, however, is so strong at times that greater care than is normally necessary must be taken to ensure the effective depression of the sphalerite and pyrite, too much of which may otherwise come up in the lead concentrate.
The normal range of the reagent consumption in the lead flotation section is approximately as follows:
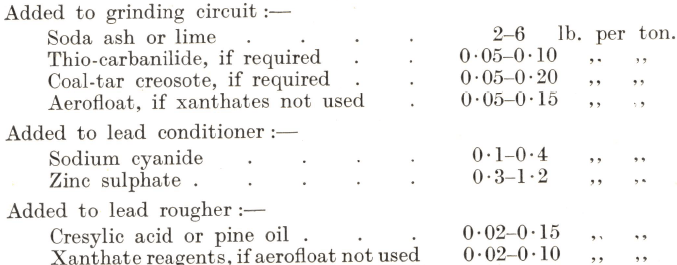
It is the usual practice for the first stage of flotation to be carried out in as thick a pulp as practicable, since the reagents constitute the largest single item of cost and their consumption depends to a great extent on the proportion of water in the pulp. The W/S ratio is seldom allowed to fall below 3/1 and is frequently as high as 1½/1. It is often possible to obtain a thick enough overflow direct from bowl classifiers, especially when grinding a dense heavily mineralized ore to a coarse or medium size, but when the classifiers cannot be run to give a fine enough product in a pulp of the desired density, it is advisable in the interests of economy to instal a thickener between the grinding and flotation sections to reduce the dilution. Sometimes, however, the presence of a thickener at this point allows the reagents too long a contact with the pulp to the detriment of flotation, in which case it should be placed between the lead and zinc flotation sections, delivering its product to the zinc conditioner. Such a circuit effects certain economies, due to the fact that any reagents contained in the overflow water of the thickener are returned with it to the first stage and can be utilized again.
The tailing of the lead flotation stage passes direct, or, as explained above, through a thickener, to the zinc conditioner, where copper sulphate is added to activate the zinc minerals. Lime is generally introduced at this point to depress pyrite and to maintain the alkalinity of the pulp at the required pH value, usually between 8.0 and 9.0. Soda ash is not often employed, as it is more expensive than lime and has no retarding effect on the flotation of pyrite. The addition of frothing and promoting reagents is made in much the same way as for the flotation of zinc ores described in the previous paragraph. The reagent consumption, however, is somewhat different, the normal range being approximately as follows :—
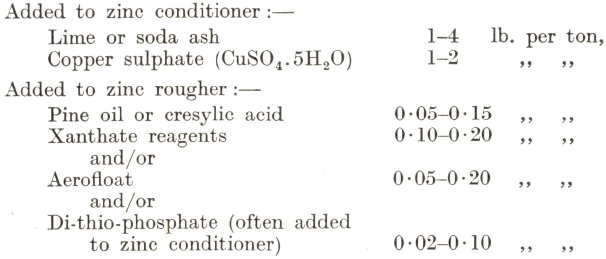
If a pyrite concentrate is required, this can usually be obtained by sending the tailing of the zinc flotation section direct to a third stage, where the iron sulphides are floated with the addition of 0.05-0.15 lb. per ton of pine oil and 0.1-0.2 lb. of ethyl and/or amyl xanthates, the latter reagent being specially suitable for the purpose.
Plants designed for the treatment of lead-zinc and lead-zinc-iron ores are more frequently equipped with sub-aeration machines than with any other type. There appears to be no reason, however, why air-lift and pneumatic machines should not give equally good results, except when prolonged agitation is necessary for successful flotation.
As a general rule, a single stage of cleaning is sufficient in the lead flotation section, and circuit 2, 6, or 7 will usually be found to give satisfactory results, since vigorous scavenging is not often necessary at this point. Two stages of cleaning and a scavenging machine following the rougher are generally needed in the zinc flotation section (circuit 4, 10, or 11). A clean pyritic concentrate, should one be required, can often be made in one machine without cleaning (circuit 1 or 5); otherwise circuit 2 or 6 is employed, a second cleaning stage and a scavenging machine seldom being necessary.
Source: This article is a reproduction of an excerpt of “In the Public Domain” documents held in 911Metallurgy Corp’s private library.
