Table of Contents
Leonardite is a coallike substance, similar in structure and composition to lignitic coal and believed to be derived from lignitic coal by the process of natural oxidation. Leonardite is little known outside lignite-producing areas and has been developed commercially only tn a minor extent. The higher oxygen content and less compact structure of leonardite, compared with lignite, make it undesirable as a fuel but indicate that it has potential as a source of chemicals and for other nonfuel uses. It is a convenient source of humic acids. The United States has a large natural reserve of leonardite; furthermore, it can be produced in the laboratory under controlled conditions.
The significant elemental difference between leonardite and lignite is the oxygen content (leonardite, 28-29 percent; lignite, 19-20 percent). The higher oxygen content of leonardite is due entirely to a larger number of carboxylic acid groups. This fact explains the 17-fold increase in alkali solubility of leonardite. Spectral studies indicate that the material is mixed salts of humic acids.
Interest in the use of low-rank fuel reserves in the United States has directed attention to certain side products, of which leonardite is a major item in the lignite field. Frequent requests for information regarding this little-known material led to the preparation of this report, in which are assembled, in convenient form for interested parties, the small amount of available data and results of current investigations. Limited commercial development of leonardite has been a reality for several years, but details of development have been the exclusive property of the companies involved, and the potential of the material has not been widely known. The information contained in this report, although meager, may indicate new uses or prompt further investigation; if so, this report will have served good purpose.
Leonardite Occurrence and Properties
Leonardite is a soft, earthy, medium-brown, coallike substance associated with virtually all lignitic outcrops in North Dakota, and it is mined commercially from the Harmon bed in Bowman County. It is frequently referred to as “slack” because of its texture, however, the term “leonardite,” after A. G. Leonard, first director of the North Dakota Geological Survey, is finding common usage. Usually, the material occurs at shallow depths, overlying or grading into the harder and more compact lignite. The seam mined in Bowman County is about 6 feet thick and lies 8 to 35 feet below the surface. Other large deposits are known, notably in Divide County, N. Dak., and similar material of an earlier geologic age occurs in Texas.
The scarcity of information on leonardite is due partly to its limited use (less than 1 percent of the total annual output of lignite) and partly because it is not a distinct mineral. The stratigraphic occurrence of leonardite, its variable properties, and the ease with which normal lignite may be converted to material of similar properties by oxidation in the laboratory suggest that these deposits result from protracted weathering of lignite beds by atmospheric oxygen and/or ground waters under oxidizing conditions. Leonardite has been described as a precursor of lignite, gel like samples similar to dopplerite have occasionally been found. Pure pre lignitic material probably would not survive to duplicate the geologic position in which the larger masses of leonardite are found, but this possibility may not be entirely discounted. The question, however, is purely of academic interest. Leonardite has been limited to use as a dispersant and for viscosity control in oil-well drilling muds , as a stabilizer for ion-exchange resins in water treatment , and as a source of water-soluble brown stain for wood finishing. Experimental work has been conducted on its use as a soil conditioner and fertilizer, This use is dependent upon its adsorptive and moisture-retaining characteristics.
Three types of leonardite have been described. Type 1 is a black colloidal material that swells to several times its original volume in water and dissolves in alkali hydroxides, leaving almost no residue. It is analogous to what has been described in earlier editions of Dana’s “System of Mineralogy” as native humus acid. It gives dark-brown, rich-colored solutions in alkali and may be precipitated as a light-brown colloid with acids at a pH of 4 or lower. Type 2 is a mixture of type 1 leonardite and lignite. Type 2 is found wherever a lignite seam is overlaid with less than 8 feet of water-precipitated product from type 1. It is fine-grained, colloidal, and intimately intergrowm with gypsum. The commercially mined leonardite is probably this type, whereas the leonardite from Divide County appears to be type 2.
Bureau of Mines proximate and elemental analyses of three leonardite samples, given in table 1, indicate the similarity of materials from different locations. Comparable analyses of a typical lignite are included to demonstrate the close relation and probable origin of the leonardite. The example of North Dakota lignite and Leonardite were carefully collected column samples representative of the seams. The history of the Texas sample is unknown, but it appears to be a dried sample of run-of-mine coal. The greater nitrogen content of this material is further reflected in an appreciable increase in the amount of extractable waxes, indicating a canneloid antecedent in contrast to the humic precursors of the North Dakota samples. The excessive ash content of the Texas leonardite may be due largely to accidental inclusions; however, increased adsorptivity, resulting from more advanced weathering, could account for part of the inorganic constituents. The sample of oxidized lignite was prepared in a Bureau laboratory by oxidizing lignite in air at 150° C.
Present Investigations
Solubility characteristics, chemical and spectral properties, and results of microscopic examination indicate that the leonardites are essentially salts of humic acids admixed with mineral matter such as gypsum, silica, and clay. The two North Dakota leonardite samples are being examined on a modest scale at the Grand Forks Lignite Research Laboratory as to the content and character of the humic acids. The humic acids have been variously defined but are perhaps best described by Oden as yellow-brown to black-brown substances of unknown constitution, formed in nature by decomposition of organic materials under atmospheric influence or in the laboratory by chemical action. They can split off hydrogen ions and form typical salts with strong bases and usually are insoluble in water, soluble in alkali, and reprecipitated by acid. Humic acids have received considerable attention, particularly the so-called regenerated humic acids obtained by oxidation of coals. It is generally conceded that the investigator is dealing with a collection of closely allied hydroxycarboxylic aromatic compounds, probably associated by hydrogen bonding and = representing species ranging in molecular weight from about 300 to as high as 4,000.
Leonardite represents a convenient and commercial source of regenerated humic acids produced under the mildest conditions. The investigation of these acids is a logical approach to coal-constitution studies and may indicate attractive features for increased commercial development of leonardite.
The initial investigations by the Bureau of Mines at Grand Forks were to determine the amounts of humic acids recoverable from leonardites, the factors influencing the amount recoverable, and the ash content of the recovered humic acids. Sodium hydroxide, sodium carbonate, or a mixture of sodium carbonate and lime were the most suitable reagents and had about equal effect. The percentages of humic acids recovered with these reagents were high and essentially equal. Yields were slightly lower with ammonium hydroxide. The concentration of the alkalis could be varied considerably without appreciably affecting the humic acid yield. When the ratio of water to leonardite was increased during extraction, the ash content of the recovered humic acids was lowered. Extraction temperatures had little effect on the yields of humic acids, although the yields increased slightly with alkali concentration of about 2.5 N at temperatures near the boiling point. Organic solvents were rather ineffective, unless the leonardites were first treated with acids. For example, later studies showed that the solubility of leonardite in acetone-water solution is directly proportional to the quantity of acid used in the pretreatment of the leonardite. The highest yields achieved with organic solvents were those in which a mixture of alcohol and benzene was used and indicated the presence of waxy material in the leonardites.
Experimental
Twenty-gram samples of the lignitic materials listed in table 1 were extracted with benzene-alcohol (minimum boiling mixture) in a Soxhlet apparatus, using a glass thimble with a fritted filter disk. The extraction was carried out until the solvent mixture remained colorless. The extract was then freed of solvent and was weighed. This material was assumed to be the total extractable wax. Results are shown in table 2.
Forty-gram samples of each lignitic material were stirred with 500 ml of 1 N NaOH for 1-½ hours at room temperature; insoluble matter was separated by centrifuging. The humic acids were separated by acidifying the supernatant liquid with HCl, were washed with water, and were recovered by centrifuging. The solid material was dried overnight at 105° C.) was washed several times with water containing a few drops of HCl, was recovered by centrifuging, and was dried at 105° C. The yield of humic acid was calculated on the basis of recovered solids. Table 2 gives the results.
Where enough humic acid was recovered, an elemental analysis was run on the recovered material. Table 2 also gives these results.
Functional group analyses were made on the leonardite from Bowman County, N. Dak. Carboxyl and hydroxyl determinations were made by Ubaldini’s method, carbonyl determinations by the method of Stracht and Brandl and methoxyl determinations by the Method of Viebock and Schwappach. Table 3 gives the results.
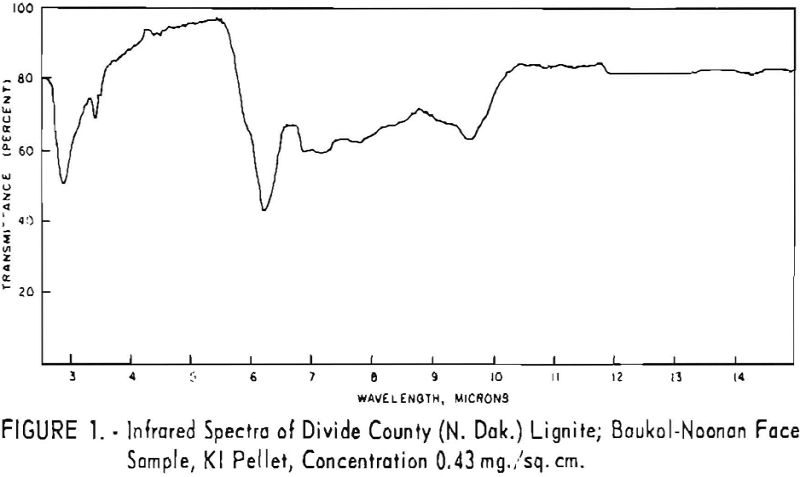
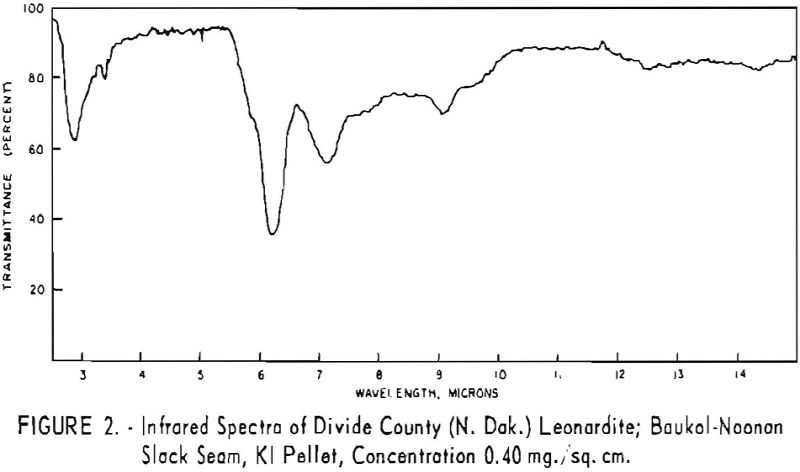
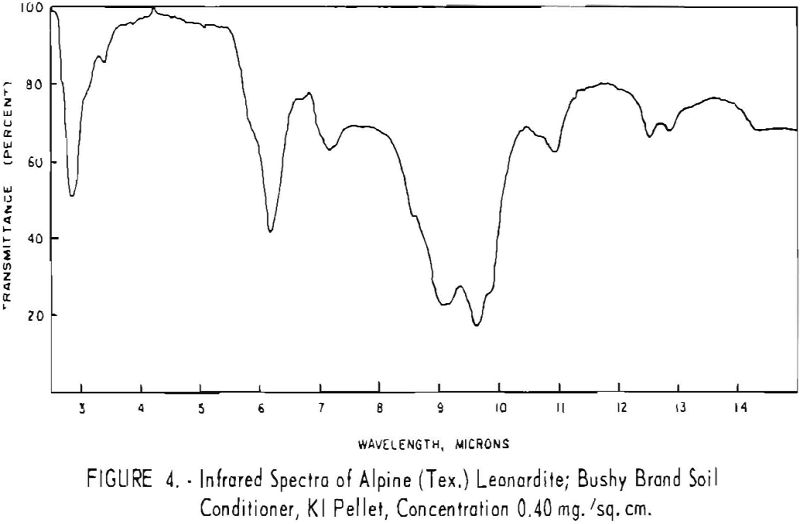
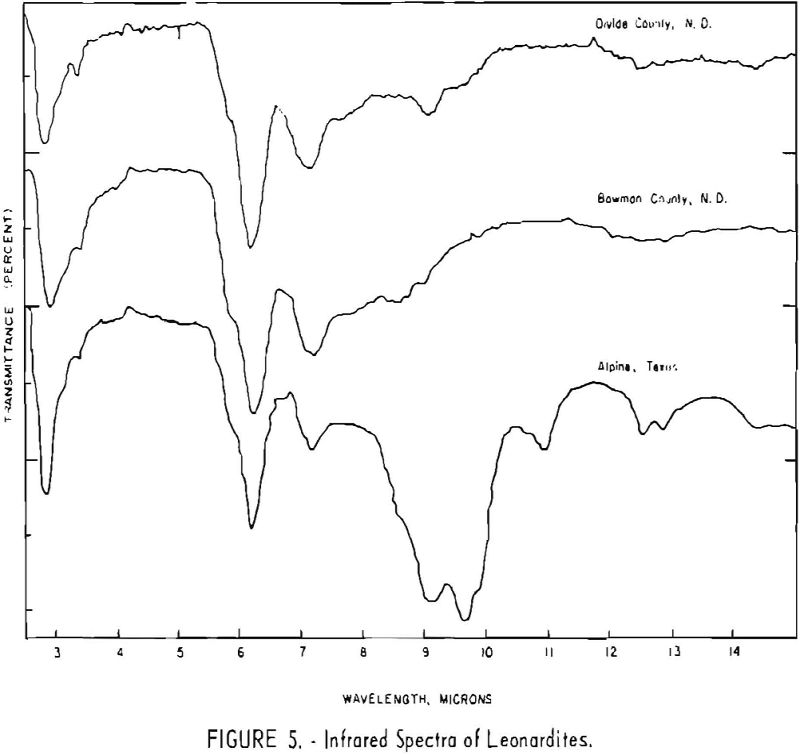
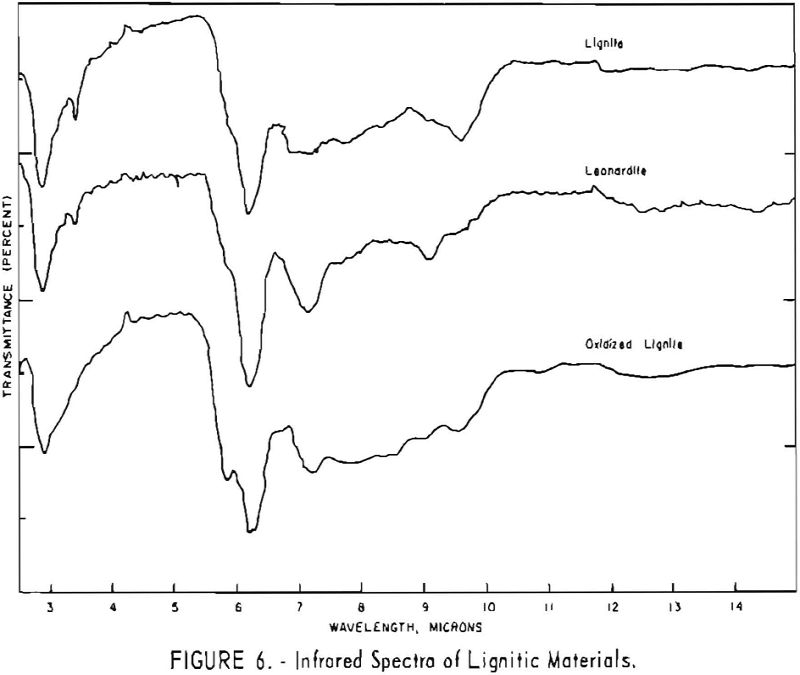
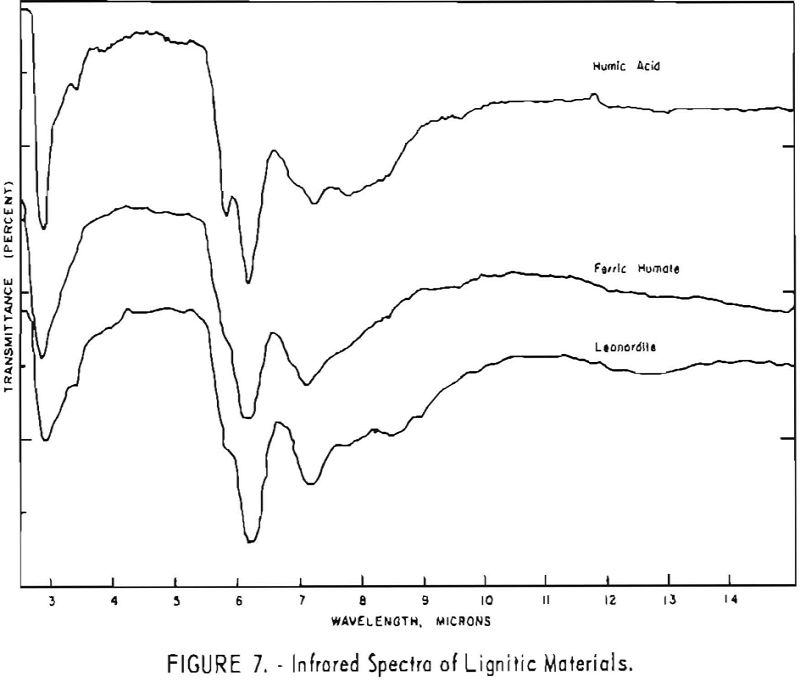
Finally, infrared spectra were obtained on potassium iodide (KI) pellets prepared with each of the solid lignitic materials already described. The spectra, shown in figures 1 through 7, were recorded on a Beckman IR-4 double-beam spectrophotometer using NaCl optics. The ferric salt of humic acids (fig. 7) was prepared by precipitating humic acids from an acetone-water solution with ferric chloride.
Discussion
Aside from the increased nitrogen content of the Texas sample (table 1), there is no significant elemental difference in the three leonardite materials; the range in sulfur content of the leonardites is usual among lignites of the North Dakota area. The slightly higher oxygen and lower carbon content of the Texas sample indicates more weathering; however, the increased nitrogen content points to a greater proportion of protein material in the formative stages of this deposit (more marine life, insects, or both). The higher percentage of benzene-alcohol extractables (table 2) shows that the Texas sample contains

more of the decay-resistant plant remains, such as pollen, spores, leaf cuticles, and other waxy and resinous material characteristic of canneloid coals.
Structural examination, on the other hand, indicates that the differences between the North Dakota and Texas samples are much more significant. The first difference, is the amount of acidic material present (soluble in 1 N NaOH). The lower figure tor Texas leonardite could be either decarboxylation due to a more advanced state of weathering (oxidation) or less oxidizable functions in its lignitic precursor because of the greater geologic age of this deposit, hence more advanced coalification. The comparatively narrow absorption band at approximately 2.8 µ in the infrared spectra (compare figs. 1 through 4 and fig. 5) contributes to this conclusion. This means also that more of the oxygen in the Texas sample is present in phenolic hydroxyl groups.
The principal bands in the infrared spectra of the leonardites are those at 2.8 to 4 µ (phenolic and carboxylic OH), 3.4 µ (aliphatic CH), 5.8 µ (carbonyl), 6.2 µ (conjugated carbonyl or aromatic) (2), 7.2 µ (CH2 or CH3 or ionic carboxyl) (2), and several bands at 8 to 10 µ attributed to ethers. Assignment of the bands at 12.4 and 12.8 µ is uncertain, but they may be due to condensed aromatics or substitution in the benzene ring. The peroxide structure has been suggested for the band at 11 µ. These bands are comparatively strong in the Texas leonardite, and as other evidence, indicates that this sample is from a more advanced stage of coalification the bands are probably due to condensed ring structures. The strong; band at 9 µ in the Texas leonardite could indicate mixed ether, and that appearing in the Divide County and Texas samples at about 9.6 µ has been ascribed to monoaryl ethers. However, it is very possible that both of these absorption peaks are strengthened by the high silica content of the ash of the Texas sample.
Essentially, these bands are also present in the spectra of lignite and oxidized lignite. The most noticeable differences between the leonardites and lignite are the decrease in intensity of the bands at 2.8 µ and 3.3 µ, the increase of the shoulder at 5.8 µ, and the sharpening of the band at 7.2 µ. Oxidized lignite shows the same changes, with a strong peak at 5.8 µ This peak is also strong in the spectra of the humic acids (fig. 7) but is greatly weakened and/or shifted in both the leonardite and the salts of the humic acids. The intensity always decreases in changing from the humic acids to their salts. Calcium and iron salts show a decrease in intensity at 5.8 µ, whereas sodium salts show a decrease not only at 5.8 µ but also at 2.8 µ. These changes might be expected if calcium reacted only with carboxyl groups and sodium reacted with both the carboxyl and the hydroxyl groups. Both changes are noted in the spectra of the North Dakota leonardites and indicate that these materials are mixed salts of humic acids.
Fractions of the gross humic acid.s that have been separated by solvent extraction, chromatography, or electrophoresis show marked changes in tho relative intensity of the bands at 2.8, 5.8, and 6.2 µ. These differences support the view that the humic acids are a group of closely related materials which differ in the degree of polymerization or in the number of carboxyl and hydroxyl groups in each molecular unit. It has also been determined that the methoxyl content of the various fractions differs somewhat. This may also account for the differences in solubility and some of the variations in the infrared spectra.
Any attempt to derive a formula for leonardite would have to be made with the understanding that it is not an individual compound but, rather, a mixture of several similar types. From the elemental and functional group analyses the formula for leonardite was calculated to be:
C140H126O5(COOH)17(OH)7(CO)10(OCH3).
The molecular weight would be approximately 3,080 and the equivalent weight 128. This formula indicates only that the leonardite molecule is not highly condensed, although some of this material may also be present. The formula for a typical lignite, on the same basis, was calculated to be:
C192H148ON3S(COOH)10(OH)10(CO)9(OCH3)
where the molecular weight is approximately 3,450 and the equivalent weight about 340.
