Table of Contents
Coals ranging in rank from lignite to anthracite, cokes carbonized at temperatures ranging from 520° to 1,180° C., and synthetic polymers have been oxidized by the standard method, that is, by boiling in 8-normal nitric acid. Some of these materials were also oxidized by one or two modifications of the standard method. Although the fusain contents of coals so determined are considered to be related to the fusain contents as estimated petrographically, there are a number of probable sources of error that warrant careful consideration. These include:
- Errors in extrapolation due to departure from linearity of the plotted data;
- Changes in chemical composition of fusain during oxidation; and
- Errors caused by the particular strength of acid selected for oxidation.
These errors are important enough that the results should be regarded as first approximations. However, petrographic estimates of fusain contents are also uncertain, and the simplicity and convenience of the chemical method may warrant its selection for certain applications.
Experimental Work
Description of Method
The method was essentially that described by previous investigators. One-gram samples of minus-200-mesh coal were boiled in 100 ml. of 8-normal nitric acid for different lengths of time. After washing with sodium hydroxide, hydrochloric acid, and water, the residues were dried at 105° C., weighed, and ashed. The weights of ash were deducted from the weights of the residues, and a plot was constructed of percent residue (m.a.f.) as a function of time of oxidation. The intercept, determined by a straight line drawn through the linear portion of the data (usually results for oxidation times of 2 hr. and longer) and extrapolated to zero oxidation time, was taken as the fusain content of the sample. This procedure is referred to as standard throughout this report for convenience in discussion.
Two modifications of the above procedure were also used. In the first, the dried residue was heated in a covered, previously ignited 25-ml. filter crucible, by placing it in a sand bath preheated to 400° C. in a muffle furnace. A thermocouple just beneath the surface of the sand bath was used to monitor the temperature. After 15 min. the sample was cooled, weighed to determine volatile matter, and ashed in the usual manner.
In the second modification, 300 ml. of 1-normal nitric acid was substituted for the 100 ml. of 8-normal acid used in the standard method; all other steps were carried out in the usual manner.
Samples
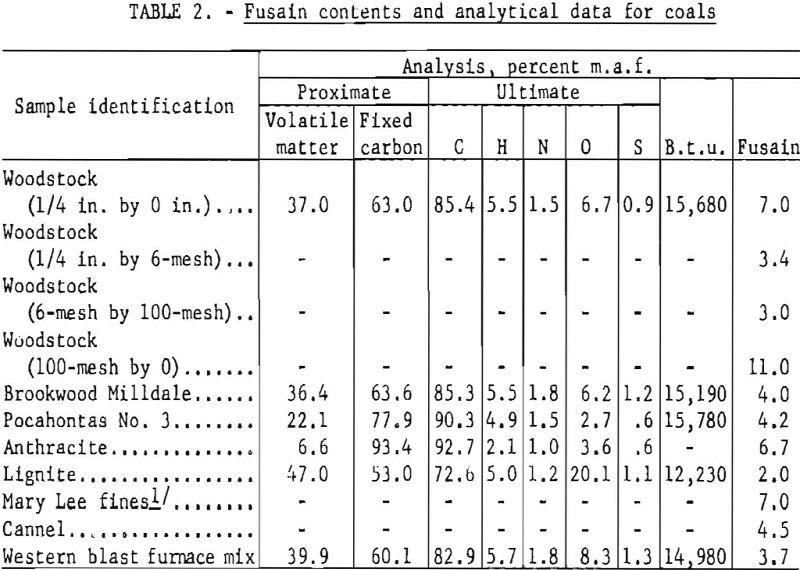
The coals tested varied widely in rank and origin. The Brookwood and Milldale coals were both of high-volatile rank; they occurred one immediately above the other in the Warrior field (Alabama) and were mined simultaneously. The Cobb and Black Creek coals were of high-volatile rank and occurred in the Warrior field. The Mary Lee coal was of borderline-high to medium-volatile rank and also occurred in the Warrior field. The Woodstock coal was of high-volatile rank and represented one of the few productive beds of the Cahaba field (Alabama). The Pocahontas No. 3 bed coal was obtained from a local coke works and was of borderline-low to medium-volatile rank. The origin of this sample was not definitely known. The western blast furnace mix was obtained from one of the coke works situated west of the Rocky Mountains and represented the blend used for coke production at the time of sampling. The cannel coal was hand selected from the Mason bed in the Middiesboro basin in Tennessee. The anthracite was shipped from Pennsylvania for use as a blending agent for commercial coke production. The lignite was shipped from North Dakota for experimental purposes.
For most of the coals tested, the test samples consisted of representative portions crushed to pass a 200-mesh screen. However, samples enriched in fusain were obtained by microscopically inspecting individual lumps of coal and manually removing that portion adjudged to consist principally of fusain. Before being crushed to 200-mesh, this material was separated in a float-sink bath with carbon tetrachloride and benzene, and the sink portion was taken to represent substantially pure fusain. The separating gravities were 1.38 and 1.30, respectively, for the Black Creek and Cobb coals.
The cokes were prepared by placing a combustion boat containing 10 of coal into a precision-type tube furnace through which nitrogen was passed to prevent oxidation. The furnace temperature was kept substantially constant except for the first few minutes. After about ½ to 1 hr., the samples ceased to lose weight and were removed from the furnace, cooled, and crushed to pass a 200-mesh screen.
Samples of plastics were stock items available at the laboratory. They were prepared by machining, followed by grinding or hammer-milling to the desired particle size. Although coal-analysis procedures are not intended for use with plastics and therefore may give misleading results, two of the samples were subjected to proximate and ultimate analyses, and comparative percentage compositions were obtained as follows: Methyl methacrylate, volatile matter 99.3, moisture 0.6, fixed carbon 0.0, hydrogen 8.3, carbon 61.2, nitrogen 0.1, oxygen 30.3, sulfur 0.0, and ash 0.1. Phenol-formaldehyde, volatile matter 76.4, moisture 4.2, fixed carbon 19.2, hydrogen 6.8, carbon 59.7, nitrogen 0.5, oxygen 32.8, sulfur 0.0, and ash 0.2. Samples of polyethylene and polystyrene contained only traces (around 0.1 pct.) of ash.
Results
Application of Standard Method To Selected Coals
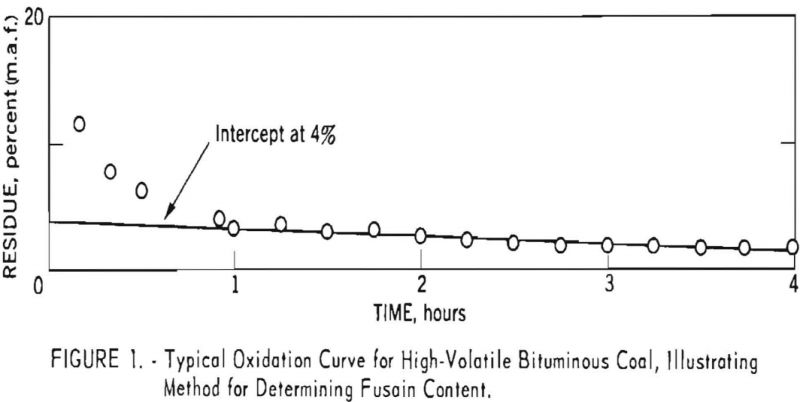
Figure 1 presents results obtained for a 60-40 mixture of Brookwood and Milldale coals by the standard method. Interpretation of the data is based on the following assumptions:
- The nonfusain constituents are oxidized far more easily than fusain and therefore are removed quickly and completely.
- The fusain residue undergoes oxidation slowly and at such a rate that a plot of oxidation time versus percent residue appears as a straight line on rectangular coordinates. The Intercept determined by extrapolating to zero oxidation time is equal to the percent of fusain in the original sample.
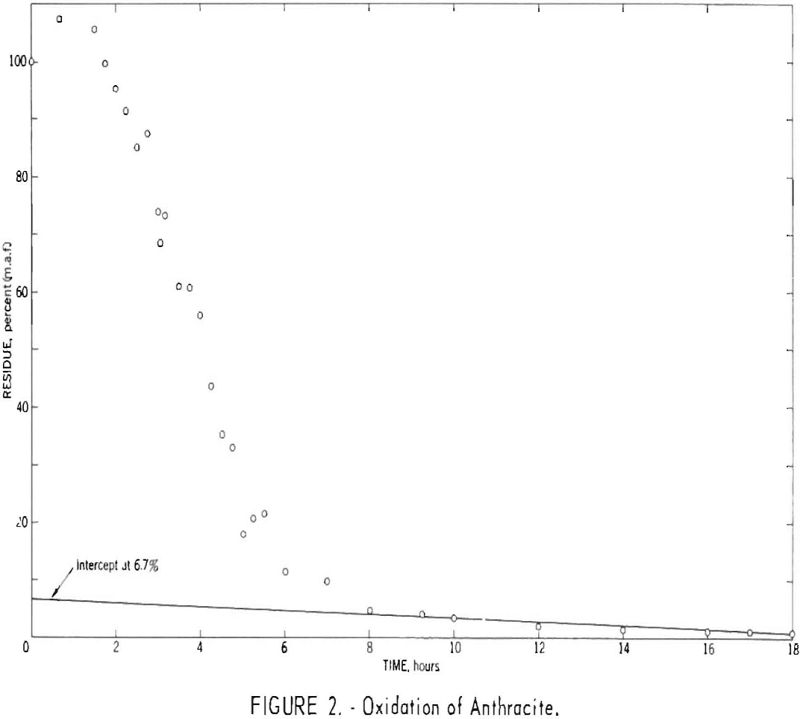
Figure 1 indicates that the first assumption holds, at least as a close approximation, for this particular coal. However, results for other coals have indicated that the time required to remove the more reactive constituents increases with rank and in some instances exceeds the 3-hr. maximum suggested by earlier investigators. Figure 2 presents results for an anthracite for which an oxidation time of approximately 10 hr. was necessary to remove the more reactive constituents. Low-volatile coals tested to date have required oxidation times of at least 3 hr.
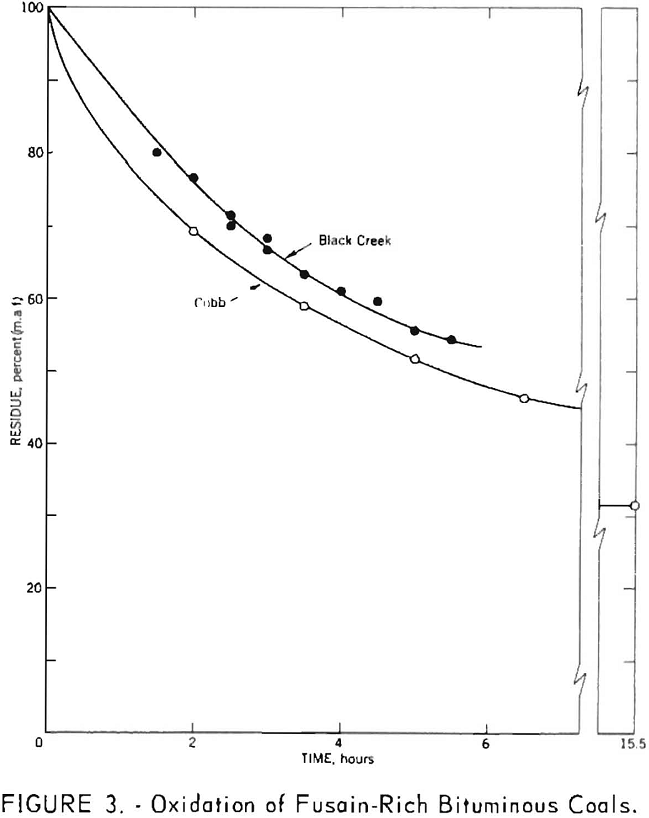
Although the second assumption also appears to hold as a reasonable approximation, the normal range of values is so small that deviations from linearity would be difficult to detect. To examine this assumption more thoroughly fusain-rich samples of Cobb and Black Creek coals were subjected to nitric acid oxidation. The results, shown in figure 3, indicate a departure from linear behavior for both samples. Since linear extrapolation over short ranges of data probably does not introduce large errors, this curvature does not invalidate the method. It does, however, suggest that the extrapolated values are best regarded as approximations which may be consistently low.
Figure 2 indicates that oxidation of anthracite for short periods (up to approximately 100 min.) resulted in a gain in weight, presumably caused by an uptake of oxygen and/or nitrogen as an initial step. Extrapolation of the linear portion of the data between 2 and 5 hr. to zero time suggests a potential weight gain of 30 to 50 pct.
The maximum actual gain recorded, however, was 7.5 pct., and a sample oxidized for 2.5 hr. exhibited a combined oxygen-plus-nitrogen increase of only 15.4 percentage points or 18 pet. referred to the composition of the original sample (table 1).
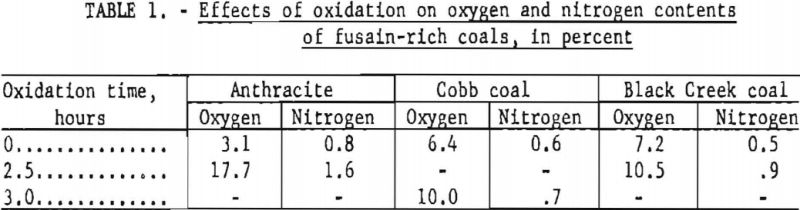
A similar initial gain in weight upon oxidation of fusain would yield extrapolated values higher than the true fusain content. However, no weight gains have been recorded for samples rich in fusain. Moreover, the shapes of the oxidation curves for these samples do not suggest the formation of any appreciable quantity of intermediate oxidation products. Comparison of analyses of the fusain-rich Cobb and Black Creek samples and their oxidation residues included in table 1 does not permit unequivocal conclusions because each sample initially contained some nonfusain constituents. However, the striking contrast between these results and those obtained for anthracite, together with the relatively low oxygen and nitrogen contents of the oxidized samples, suggests that any bias associated with a possible weight gain is small.
Fusain contents of various coals tested by the standard procedure are given in table 2.
Application of Standard Method To Selected Cokes
In connection with a study of the effects of infusible substances on the properties of cokes made from various coals, the relative resistance toward oxidation of fusain that occurs naturally in coals was compared with that of various infusible substances-such as coke fines, low-temperature char, anthracite, and petroleum coke-which are commonly added to coking blends. A sample of lampblack was included to extend the range of materials tested.
Figure 4 shows oxidation curves for cokes made from a 60-40 blend of Brookwood and Milldale coals, together with similar results for a high- temperature (1,180° C.) coke made in the Tuscaloosa oven using this same blend. The data show that the reactivity of the cokes decreased with in¬creasing temperature of carbonization over the range studied.
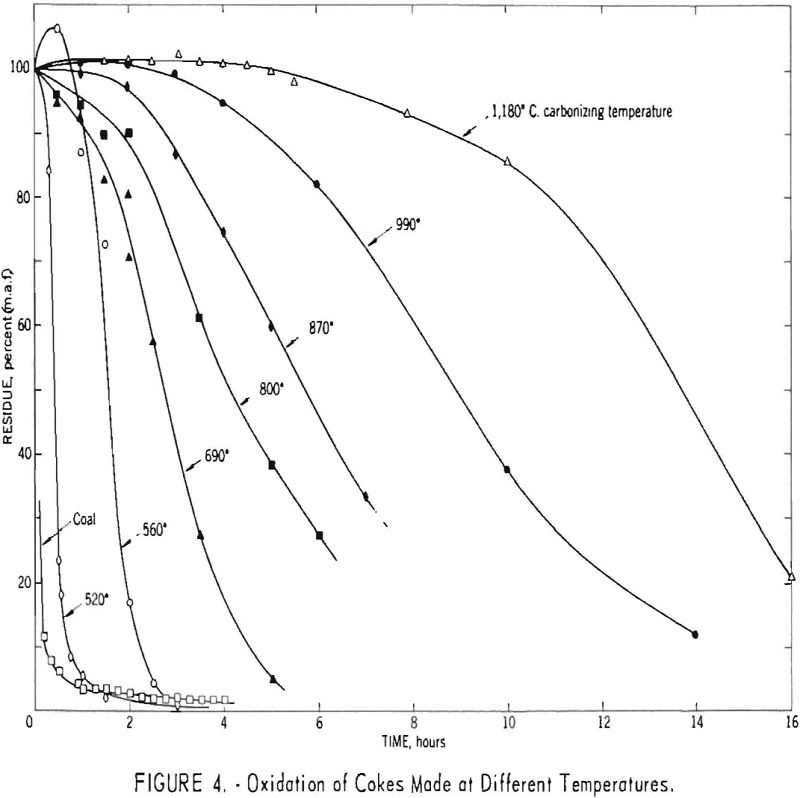
Although only a few points represent gains in weight, each curve except that for coal is characterized by an apparent induction period of varying duration, which suggests an initial gain in weight due to oxygen and/or nitrogen uptake. To obtain additional data regarding these phenomena, two cokes were oxidized for short periods and analyzed. The results, summarized in table 3, indicate a marked increase in oxygen content and volatile matter but only a slight increase in nitrogen content. The increase in volatile matter was 1.76 times the increase in oxygen content for the high-temperature (1,180° C.) coke. The corresponding ratios for the 700° C. coke were 1.61 for 2-hr. and 1.72 for 4-hr. oxidation periods. Comparison of these ration with the theoretical weight ratios of CO/O and CO2/O2 (respectively 1.75 and 1.37) tends to suggest that on heating much of the added oxygen was evolved in the form of carbon monoxide.
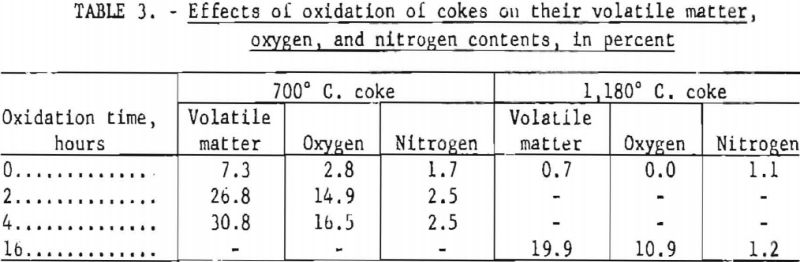
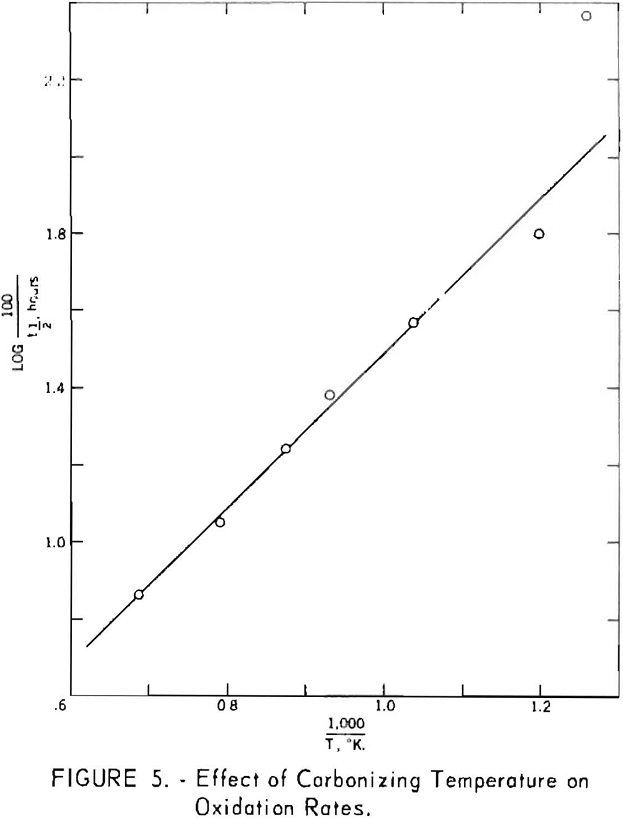
Figure 5, derived from figure 4, shows the time in hours required to oxidize 50 pct. of the various cokes as a function of the reciprocal of the carbonizing temperature on the absolute scale. The function is linear for temperatures greater than approximately 550° C. For the 520° C. coke, the time required was disproportionately short and approached that for the uncarbonized sample. These data, therefore, are indicative of changes in reactivity associated with secondary degasification occurring during the latter stages of carbonization. The apparent energy of activation determined from the slope of the line is -9.2 kcal. per mole. The assumptions implicit in the methods used to determine this value are highly questionable, however, and caution should be used in comparing it with results obtained by other methods.
Figure 6 presents oxidation curves for different types of infusible carbonaceous substances. These consist of two cokes carbonized at different temperatures, anthracite, fluidized petroleum coke, fusain-rich Cobb coal, and a commercial lampblack. The results for the anthracite and petroleum coke were generally similar to those for the 800° C. coke, and those for lampblack roughly approximated those for the 990° C. coke.
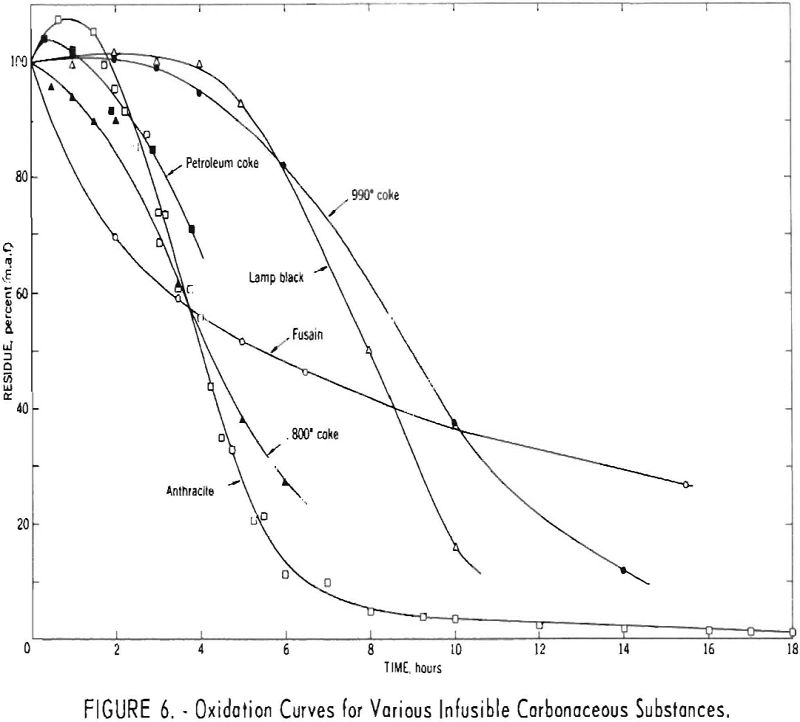
The curve for the fusain sample differs in shape from those for the other substances, suggesting that fusain is oxidized by a somewhat different route. For the other substances, the initial reaction probably involves accumulation of appreciable amounts of a relatively stable intermediate oxidation product. For fusain, the shape of the oxidation curve suggests that either this intermediate product is never formed or else it undergoes further oxidation and/or decomposition at a rate such that no detectable quantity accumulates under the conditions of the reaction.
Application of Standard Method to Selected Plastics
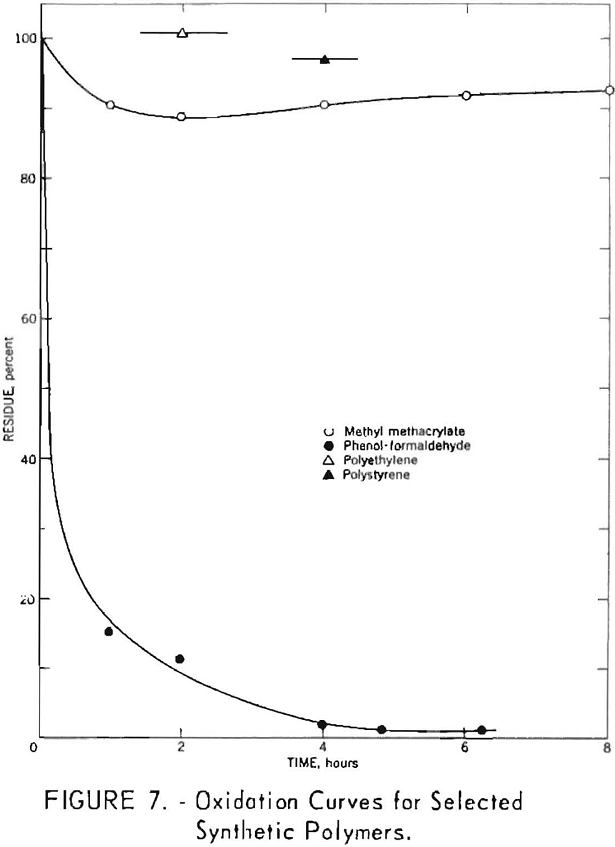
Several commercial plastics were subjected to nitric acid oxidation. Results for phenol-formaldehyde, methyl methacrylate, polyethylene, and polystyrene polymers are given in figure 7. Polyethylene melted during oxidation, and the residues from methyl methacrylate and polystyrene showed evidence of incipient fusion. Each of these materials exhibited a high resistance toward degradation by boiling 8-normal nitric acid. Phenol-formaldehyde was less resistant, and its oxidation curve was similar to those noted for a number of coals.
Analytical data for the phenol-formaldehyde and methyl methacrylate plastics are given in table 4; the other plastics were not analyzed. These values should be used with caution, as they were obtained by using coal-analysis procedures which may not be entirely suited to analysis of plastics.
Application of Various Modifications of Standard Method to Selected Coals and Cokes
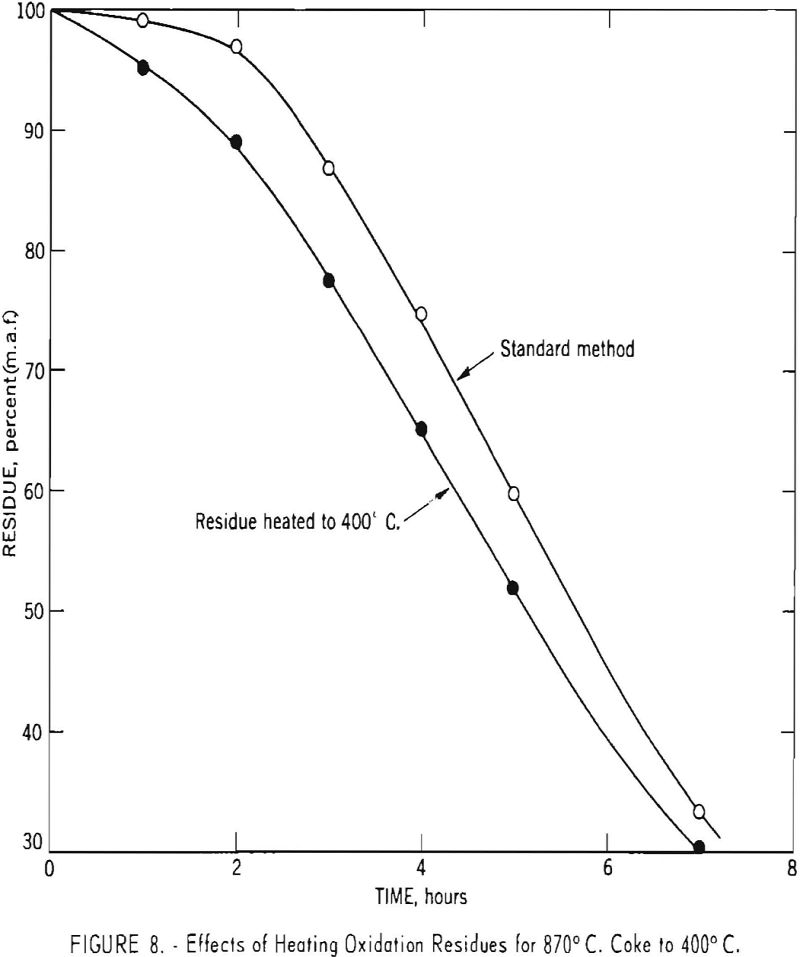
To obtain additional information relative to the weight gain during the initial phases of the process, a number of cokes-all carbonized well above 400° C.-were oxidized as usual except that the dried residue was heated to 400° C. to determine the extent of weight loss. Typical results for 870° C. coke from Brookwood-Milldale coal are given in figure 8. The volatile matter of the residue (referred to the weight of the original sample) is greatest at intermediate recovery levels and amounts to about 10 pct. For some other samples, much larger amounts of volatile matter have been noted. Thus, after a 1-hr. oxidation period, an 800° C. char made from charcoal gave a residue amounting to 102.2 pct. of the original weight and containing 42.6 pct. volatile matter.
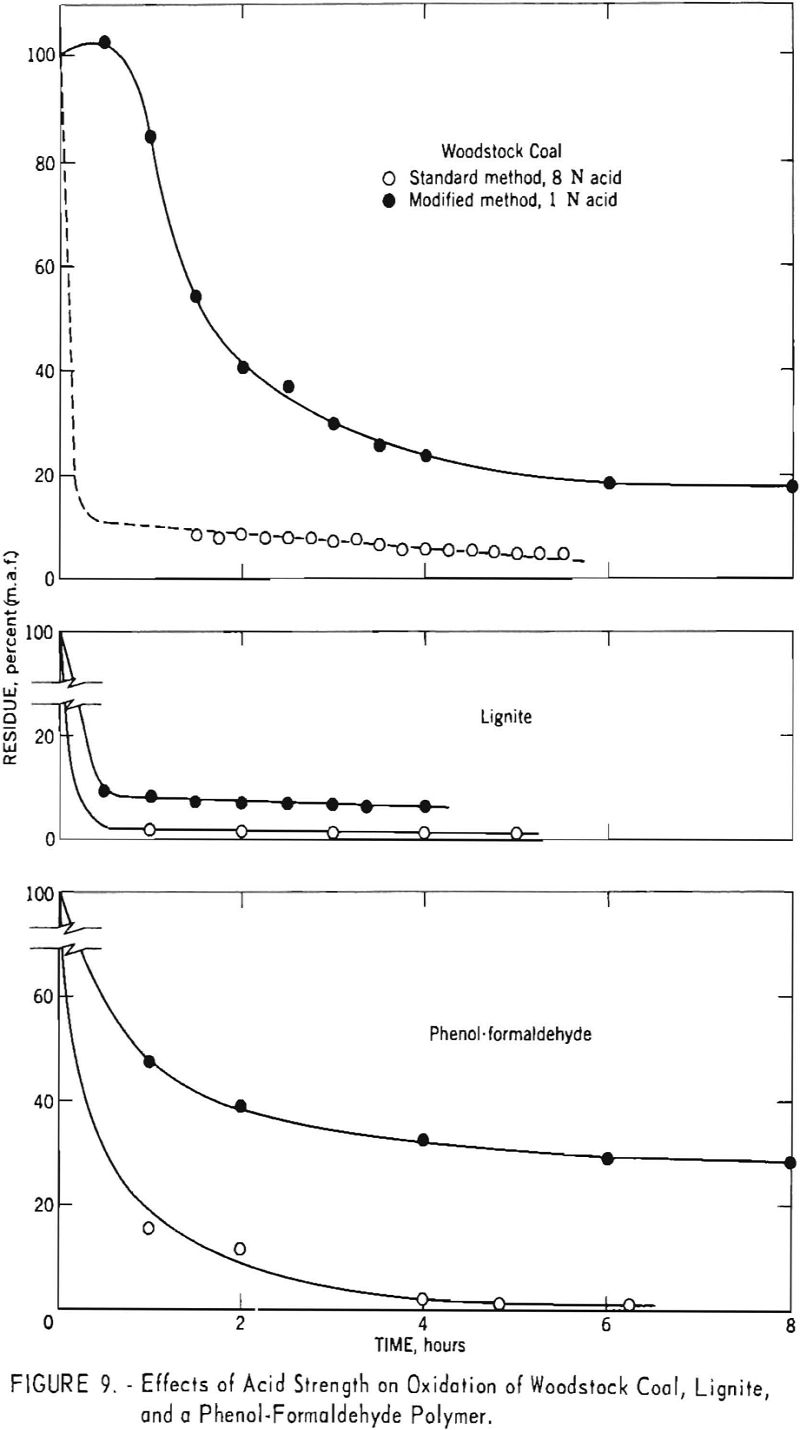
Many investigators have used different acid concentrations and/or reaction temperatures to selectively oxidize different petrographic constituents of coal. However, most procedures have involved oxidation for a fixed length of time. To determine the effects of acid concentration on oxidation rates, several samples were oxidised for different lengths of time with 1-normal acid. The reaction was carried out under reflux, and the products were worked up in the usual manner. Figure 9 presents comparative results for Woodstock coal, lignite, and phenol-formaldehyde polymer obtained with both 8- and 1-normal acids. In each Instance, the two acid strengths give similar curves except that the linear portion of the plot occurs at a higher recovery level. Thus, results for 1-normal acid appear to indicate the presence of a larger quantity of fusain than that determined by use of 8-normal acid for the coal and lignite. The results for the phenol-formaldehyde polymer likewise indicate that the amount of oxidation-resistant material depends on the strength of acid used.