These results obtained in experiments, proving that a low percentage of copper sulphate with a variable percentage of salt, depending on the ore, gives the best results, confirm practical mill-work. I have none of my notes, taken at the time, to refer to, so have to rely solely on memory, which precludes the conciseness that is always desirable.
At that time, the plant was treating a very refractory ore carrying about 15 grams in gold and from 20 to 25 oz. of silver per ton, the bullion being about 300 fine in gold. The silver in the ore was in the form of a sulphide.
Although carrying an average of 0.75 oz. of gold per ton, free gold could rarely be seen by panning, but upon roasting a sample of the ore the gold became visible at once—a result which was discovered by chance and used to advantage later.
In order to utilize the old mill to the best advantage, it was decided to use pan amalgamation after roasting. The roasting, done in a lime-kiln, was similar to the ordinary “burning” operation. The roasted ore was trammed to the mill-bins and crushed by 10 stamps to pass a 30-mesh screen. The pulp was de-watered and fed to four 1-ton pans.
My recollection is that upon starting the mill-work, the charge contained 1.5 lb. of copper sulphate and 5 per cent, of salt per ton.
From the start the extraction was unsatisfactory and the loss of quicksilver was very large. Experiments were then carried out on a working scale. First, the quantity of copper sulphate was increased 4 oz. at a time; then it was reduced at the same rate and notes carefully recorded. To my surprise, when the charge of copper sulphate was lowered from the original 1.5 lb. per ton, the percentage of extraction increased, and at 4 oz. per ton of charge the best extraction was made. The quantity of salt was then varied, and 5 per cent, was found to give the best results with the 4-oz. charge of copper sulphate.
When I explained what I was doing to the metallurgist of a nearby plant, he asked, “ Why do you use any copper sulphate at all ?”
The nature of the ore may be judged by the fact that with only 4 oz. of copper sulphate per ton of ore treated the loss of “quick” was still 1 lb. per ton of ore treated.
In their paper, Messrs. Hofman and Hayward deal with a branch of silver metallurgy that is on very uncertain ground, both as a commercial process and as a metallurgical science ; moreover, its field is limited to few localities and to peculiar conditions. The literature on the subject is incomplete and full of conflicts. The paper under discussion clears up no disputed points; on the contrary, because of hasty generalization, its tendency is towards further confusion.
In Table I., the authors assume that each test-lot of 1,800 g. of ore contained exactly 5.499 g. of silver, and that any variation in the quantity of silver recovered, however slight, should be ascribed to variation in the time of grinding. It is barely possible that the ore was mixed so uniformly as to justify this assumption; but those of us who know how difficult it is to get uniform samples of any ore in which silver mineral is present in rich particles will have a doubt on this point.
The unlikelihood of getting absolutely uniform test-lots of ore also reflects on the data in Table II., on the effect of varying the amount of salt in a pan-charge. The authors say: “The salt series was the last one that was investigated.” This again raises a question as to whether the ore was split into test- lots at the time of sampling, or whether it was kept in bulk in such a way as to permit mechanical concentration of values before the end of the experiments.
The conclusion following Table II.: “ There is no reason, therefore, for going beyond 6 per cent, of salt,” is hardly to the point. If we assume that salt is of benefit only when in solution, it follows that the relation between the quantity of salt and the quantity of water is of more consequence than the relation between the quantity of salt and the quantity of ore. Water will hold 26 per cent, of salt at the point of saturation; then why add 180 g. of salt to only 500 cc. of water, as was done in the experiments detailed by the authors?
In discussing the results from the use of blue vitriol, the authors overlook, as so many writers have done, the chemical effect of metallic iron, an excess of which is always presented by the pan-parts and liners. It can be demonstrated that the blue vitriol ceases to remain in solution after coming in contact with the pan-iron, the copper being completely precipitated in metallic form, and being afterwards taken up by the mercury.
In every pan-charge to which salt and blue vitriol have been added, soluble chlorides and sulphates of iron and of sodium will be found. These secondary compounds do act in some beneficial way in the treatment of sulphides of silver, and a quantitative study of their effects might open up a fruitful field. The statement: “The addition of blue vitriol to the pan, as shown in Table V. and Fig. 9, has no beneficial effect whatever; on the contrary, the extraction decreases,” is apt to be misleading to the casual reader who fails to note that in the ores used for experiment practically all of the silver was in free state.
Several years ago, while in charge of a large pan-mill, I made a long series of test-runs in a 30-in. experimental pan as well as in the regular 5-ft. mill pans, sometimes substituting cement and wood for the iron pan-parts, and using nearly every common mixture of ores and reagents. Careful sampling of each test-lot of ore, both before and after treatment, was an essential feature of the tests ; and, sometimes, the samples were screened into sizes before assaying. Moreover, by taking samples from the amalgamating-pans at varying stages of the process, and then subjecting the filtrates from these samples to chemical analysis; also, by analyzing the particles of amalgam obtained from these periodic samples, some additional light was obtained. Many of the analyses were only qualitative, and on account of the closing-down of the mill for lack of ore, the investigations were interrupted before the series of tests was complete. Unfortunately, the notes of this work are not within reach. A few things, however, seemed clear:
- Iron and mercury are the most important reagents in the pan process.
- In some cases, the mercury is quickened by the addition of a small quantity of freshly precipitated metallic copper. This is one of the things ultimately resulting from the use of blue vitriol.
- Salt (sodium chloride) solution serves as a solvent and wash, rather than as a reagent, and is most valuable when hot and concentrated. It helps to keep the mercury clean. As a solvent for silver chloride, it gives metallic reducing-agents a better chance to act on mineral in that form.
- Soluble iron salts, and other secondary products that come from the use of salt and blue vitriol, are of considerable value in the treatment of ores containing silver sulphides.
- Free acids sometimes assist in the treatment of ores containing native silver sulphides, but are of no benefit with native silver chlorides, and are positively detrimental when the ore contains any lead carbonate or other oxidized lead mineral.
It is not safe to lay down any hard and fast rules, because ore from every mine has its own peculiarities, and numerous unknown factors are introduced locally, the results of which can be determined only by careful analysis, followed by painstaking working-tests. The student of this branch of silver metallurgy, who wishes to save himself much wearisome labor without being led into wrong paths, would do well to consult the works of Percy and Collins. Dr. Percy has sometimes been called the father of English metallurgical literature. His work in the field of silver metallurgy, although only a chapter in his career, commands our admiration because of the exhaustive attempts to get at fundamental principles. His own experiences, as well as the work of former experimenters, are set down in a way that all may imitate with profit and few will succeed in equaling. In beginning one of his prefaces, he says: “Of all the branches of metallurgy, that of which silver forms the subject is, in my opinion, the most extensive, the most varied, and the most complicated.”
Taking up the criticisms in the order in which they have been made :
The paper is accused not only of failing to clear up disputed points in pan-amalgamation, but of committing the fault of increasing the confusion that may still exist, in explaining the reactions that govern the process. The main object of the paper was to show that pan-amalgamation can be made to serve as a valuable typical experiment for teaching a student how to adapt a metallurgical process to the treatment of a given ore. At the same time this laboratory-experiment supplements the class-room exercise ; in addition, it interests the student, in that the results obtained are quantitative and of such a character that they can be used as a basis for large-scale treatment of the ore under consideration; and lastly, as carried out at the Massachusetts Institute of Technology, the summarizing of the results of a series of ten experiments is accomplished in a few days, before the attention of the student is diverted by other metallurgical work. The study of disputed points in panamalgamation did not come under consideration at all. In fact, if this had been the object, the manner of going to work would have taken an entirely different character. An example of students’ work along this line is given later.
The supposition in the paper that each test-lot of 1,800 g. of ore contained exactly 5.499 g. of silver is attacked, and the suggestion is made that this was improbable. As the method of preparing the ore for the tests was not given, this criticism is justified; it will disappear, however, when the manipulations are described. The lot of ore was crushed to pass a 30-mesh sieve, mixed thoroughly on the crusher floor, and sampled by fractional selection, every third shovel being reserved, until the lot was reduced to about 50 lb.; this was further cut down by means of a split-shovel, etc., to furnish two 5-lb. samples. These were assayed by crucible- and scorification-methods, and the results were corrected by determining the losses due to scorification and cupel-absorption. The ore was packed into air-tight wooden boxes, each holding about 60 lb., and stored. Whenever a box was taken out for class-work, it was emptied and the contents were thoroughly mixed. The procedure in handling the ore justifies the assumption that any uneven distribution of silver due to a possible unmixing in storage was corrected before the ore was charged into the pan, and the acceptance of the original assay, and with it the presence of 5.499 g. of silver in 1,800 g. of ore, was therefore warranted.
The third objection, that an excess of salt was used over that which the water could dissolve, holds good, and we plead guilty.
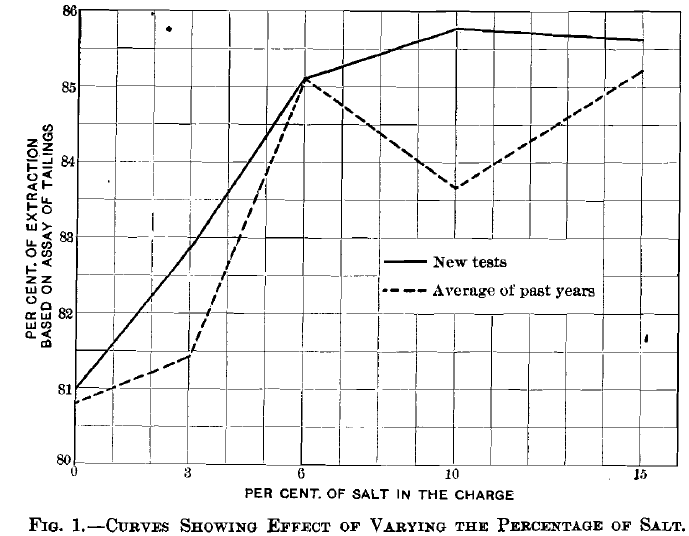
In the paper the statement was made (p. 526) that it did not seem clear why the extraction in silver, high with an addition of 6 per cent, of salt, should decrease with 10 per cent, and then rise again, and that the anomaly required further investigation. In A recent series of tests, the highest percentage of salt was given to pan No. 1 and no salt to pan No. 10, instead of having the reverse order as usual, because it was suspected that the fall in extraction might have something to do with the working of the pans. We were led to this idea, because with the style of pan formerly used in the laboratory, some pans always gave better results than others, but in the present pans no such discrepancies had been noticed, as the pulp-current always appeared to be uniform. In Fig. 1 the dotted curve represents the line given in the original paper; the full-drawn curve shows the results obtained by the last series. Here, as was expected, the extraction increased quickly with an addition of from 1 to 6 per cent, of salt, then more slowly with from 6 to 10 per cent., and remained practically unchanged when more than 10 per cent, was used. The quicker rise of the new curve and its general position above the older one are probably due to the time of grinding having been changed from 1.5 hr. to 1 hour.
As to the use of blue vitriol in an iron pan, the fact that iron precipitates copper was not overlooked; it could not well be, considering the weight and copper-content of the retort-silver obtained by these tests. Nevertheless, experiments were carried out with an addition of blue vitriol, as, according to practically all reliable literature on pan-amalgamation, this salt is one of the first reagents with which to experiment for an increase in the yield of silver. The conclusion drawn from the results, that blue vitriol had no beneficial effect, in fact decreased the extraction with the ore under consideration, is not misleading, but absolutely exact.
We may add that, in connection with the tests with blue vitriol in iron pans, experiments were carried on in copper pans of the same construction, the copper containing 1 per cent, of silicon in order to reduce the wear. As grinding in copper pans is not permissible, the pulp was previously ground to pass a 100-mesh sieve. The extraction of silver based on the tailings- assay was unsatisfactory; basing a yield on the silver recovered in the amalgam was not feasible on account of the practical impossibility of cleaning-up the pan. Work in copper pans was therefore dropped. If pan-amalgamation were a live issue, it might be worth while to try the bronze pans as used in the Franke tina process at the Huanchaca mine, Bolivia.
The results with the ore that he was treating in a large pan-mill are of interest as far as they go; they would have been more valuable if he had told us more about the character of the ore. However, he falls into the error of making “hasty generalizations,” which tend “towards further confusion” of a subject that “is incomplete and full of conflicts”— just the points that we avoided, and that he read into our text, although they are not there. In carrying on a metallurgical investigation, accurate conclusions can only be arrived at if a test or a series of tests is so planned as to determine the status of not more than one variable. As soon as you ask your experiment to give an answer at the same time to more than one question, you are sure to go astray.
We can heartily indorse the recommendation of the works of Percy and Collins on the metallurgy of silver. All the books of Percy are of permanent value, because he studied critically the metallurgical chemistry of the metals he was discussing and supplemented by original experiment any gaps that he found in his study. Even if Percy’s Silver and Gold was somewhat out of date as regards practice when it appeared, in our day we go back to it when a question as to the metallurgical behavior comes up, to see whether he has said anything about it. Of Collins’s Metallurgy of Silver we are awaiting a new edition, in which we may expect to find the new available material carefully chronicled and sifted, as is the case in the original work.
As the conclusions with regard to the treatment of a certain ore in an iron pan mention the behavior of silver sulphide, some data taken from the records of the metallurgical laboratory of the Massachusetts Institute of Technology may be given to supplement the general knowledge available at present. The leading statements regarding the behavior of silver sulphide are those of Percy, Schnabel, Adams, Huntington, and Rammelsberg. Percy says that silver sulphide subjected to the action of an aqueous solution of sodium chloride with access of air gives no silver chloride. Schnabel records that silver sulphide is slowly decomposed by quicksilver, with the formation of mercuric sulphide, and that the decomposition is more rapid in the presence of iron, especially upon heating. J. M. Adams found that an addition of salt to the pan did not chloridize the silver in the ore, but had a stimulating effect upon the extraction, as the yield in silver was always higher when salt was present than when it was absent. Huntington’s work shows that quicksilver extracts from silver sulphide in the presence of sodium chloride, sand, and water about 87 per cent, of the silver, while the yield is only 29 per cent, in the absence of salt. Rammelsberg found that silver sulphide was decomposed only slowly by quicksilver in the presence of water at 100° C.; the yield of 12 per cent, was increased rapidly to 95.2 per cent, with the addition of iron.
In the experiments, the finely-divided silver sulphide was prepared by fusing together silver, sulphur, and carbonate of potash and leaching the cake in the water. It may be added that the sulphide obtained is a slimy mass, which settles very slowly and is extremely difficult to wash completely. Some silver sulphide was prepared by precipitation from a nitrate solution by means of hydrogen sulphide. No difference was noticed in the behavior between the sulphides prepared in the dry and in the wet way. An artificial ore was made up by mixing 60 per cent, of quartz, ground through a 40-mesh sieve, and 40 per cent, of fire-clay. This proportion was chosen, as it proved to furnish the most satisfactory pulp-current. Enough silver sulphide was added to the mixture to furnish an ore assaying 100 oz. of silver to the ton.
The pan-charges were made up of 1,200 g. of quartz, 800 g. of clay, 800 g. of water, and 400 g. of mercury. The time of amagalmating was 90 min., and the temperature of the pulp about 70° C.
- Amalgamation in a copper pan with hydrant-water gave 65 per cent, extraction ; with hydrant-water and 1 per cent, by volume of H2SO4, sp. gr. 1.84, gave 68 per cent, extraction; with hydrant-water, and salt to the amount of 5,10, and 15 per cent, of the weight of the ore, gave, respectively, 84, 94, and 96 per cent, extraction.
- Amalgamation in an iron pan with hydrant-water, and salt to the amount of 5, 10, and 15 per cent, of the weight of the ore, gave, respectively, 37, 70, and 63 per cent, extraction. The results show that acidifying the pulp increases slightly the yield in silver, that an addition of salt has a very favorable effect, and that copper acts more energetically than iron.
- Amalgamation in a copper pan with distilled water, the quartz and clay having been first digested with hot distilled water until all soluble salts had been extracted—was carried on to see whether the decomposition of the silver sulphide was due to chemical or to galvanic action. The electric conductivity of the water after a charge of water, mercury, and quartz- clay mixture had been worked was found to be 28 times as great as the conductivity of the original distilled water, showing that the preliminary digestion had not been sufficient, and that in the amalgamation some salts had gone into solution; an addition of silver sulphide to the pulp and working again under standard conditions, increased the conductivity only three times above the last figure, proving that only a small amount of the sulphide had been rendered soluble. The conclusion is that the decomposition of silver sulphide is mainly chemical and only slightly electrolytic.
- Amalgamation in a copper pan with hydrant-water, salt to the amount of 10 per cent, of the weight of the ore, and an addition of 5 and 10 per cent, of galena, gave, respectively, 88 and 85 per cent, extraction; 5 and 10 per cent, of chalcopyrite gave 80 and 85 per cent, extraction, showing that the presence of these base-metal sulphides has a harmful effect.
The data furnished are not sufficiently complete to settle definitely the manner and the rate of decomposition of silver sulphide, but they show the trend of the reaction and indicate the yield that may be expected under conditions resembling those of the experiments. The subject has not been carried further, as the interest in pan-amalgamation has decreased in recent years. The manner of carrying on experimental work of this kind retains its original value.
Pan- Amalgamation : an Instructive Laboratory Experiment. Discussion of the paper of Messra. Hofman and Hayward, p. 382.
