Zirconium diboride of a quality comparable to that produced commercially from zirconium dioxide may be synthesized directly from zircon.
The quantities of residual carbon and silica are not excessively high, but it is believed that some refinements in procedure may lower these percentages.
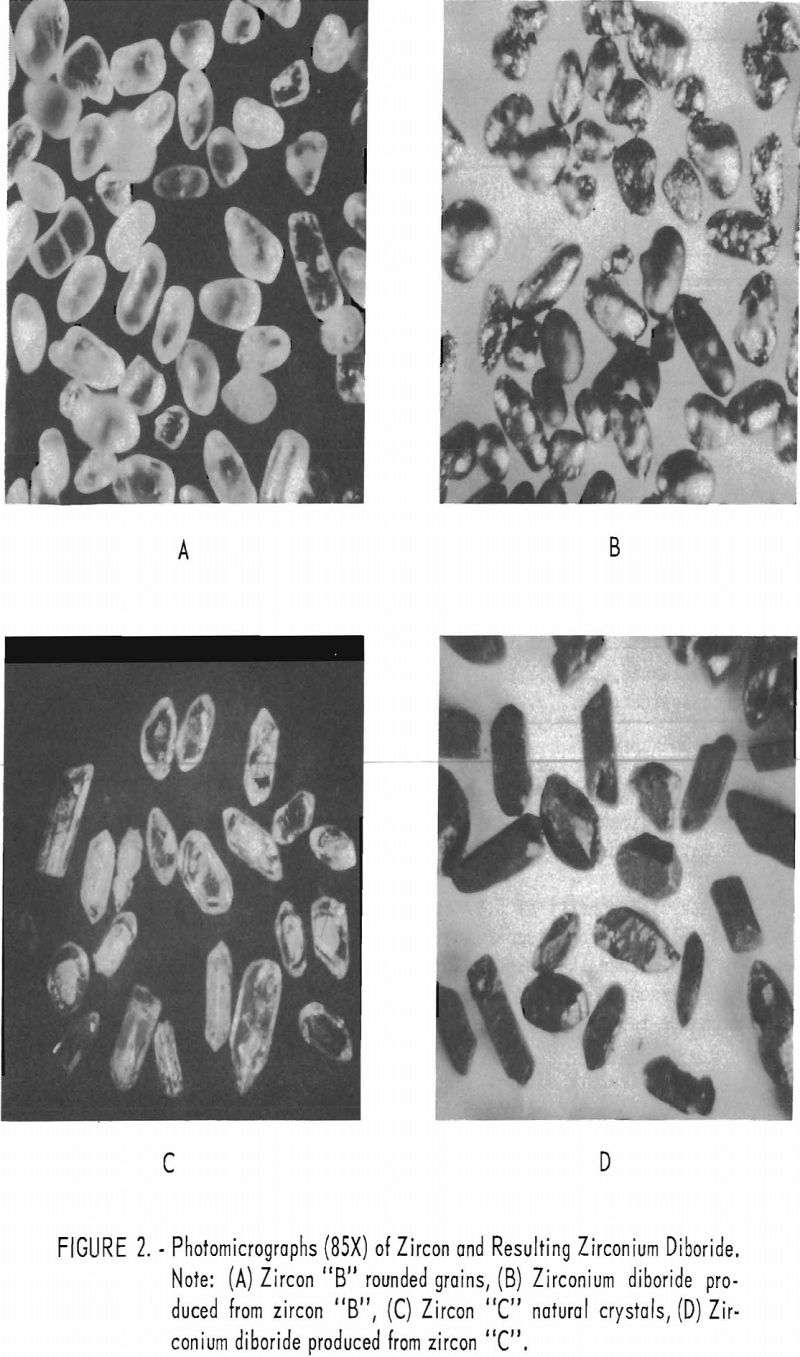
The grain size of the final product has been demonstrated to be a factor of the original grain size of the starting zircon. Because of the retention of this original grain size and crystal shape (pseudomorphic), as shown in figure 2 (B and D), it is possible that new uses for the product, such as filters for hot gases, may be developed.
The borides of the transition metals in Groups IV and VI exhibit, in addition to a certain degree of hardness, considerable resistance to heat and oxidation. They have high melting points (1,850°-3,060° C.) and low coefficients of thermal expansion and can be bonded with other materials and hot-pressed to desired shape (cermets).
Suggested uses for the borides include use in rockets and jet engines, as thermocouple protection tubes, as containers for molten metals in die casting, as flame-plated coatings on less refractory compositions to prevent erosion by hot gases, and as control rods and neutron shielding material in nuclear engineering.
The Federal Bureau of Mines investigation of zirconium diboride started in 1950 at the Boulder City Nev., Metallurgy Research Laboratory. Its purpose was to reduce the cost of raw materials for the production of zirconium diboride.
The investigation was recessed after several exploratory runs. Later the Bureau’s interest in borides developed as a result of experiments designed to produce hard and abrasive materials based on carbides. Experiments for the purpose of synthesizing zirconium diboride were begun in 1955 at the Norris Laboratory.
In commercial practice, zirconium diboride is ordinarily made by reacting the metal, the metal hydride, or the metal oxide with boron or with compounds that release boron. Methods previously reported include the following; (1) Reaction of zirconium oxide with boric oxide and carbon in a graphite crucible at 2,000° C. (2) reaction of powdered metallic zirconium and elemental boron (3) electrolysis of fused salts (4) heating of mixtures of zirconium oxide, boron carbide, and carbon in an electric resistance furnace (5) heating of zirconium oxide, lampblack, and boron carbide under vacuum, and (6) hot-pressing of mixtures of zirconium carbide and boron or boron carbide.
The theory derived from the work of others was that zirconium diboride might be made directly from zirconium silicate (zircon) without the intermediate production of zirconium oxide.
This report describes the method of synthesis and some of the results of that synthesis.
Experimental Procedures
Equipment
The equipment used in this synthesis is shown in figure 1. A is a high-frequency induction coil powered by a 20 kilowatt spark gap converter. Inserted in this coil is a closed-bottom fused silica vacuum tube, C, 25 inches long with an inside diameter of 5 inches. The graphite reaction crucible is placed inside a fused silica sleeve, which is separated from the vacuum tube by a one-fourth inch air space. The space between the fused silica sleeve and a graphite reaction crucible is packed with a heat-insulating material (Thermatomic carbon) . The fused silica vacuum chamber is closed at the top by a self-sealing water-cooled brass assembly, E, which contains a sight tube and a vacuum port. The vacuum port may also be used to supply gases for an inert atmosphere. The capacity of the mechanical vacuum pump, B, is 300 liters per minute. This pump can pump down to a pressure of 1 micron. Attempts to use an oil diffusion pump to secure a higher vacuum were found impractical because of pressure jumps occurring during various stages of the
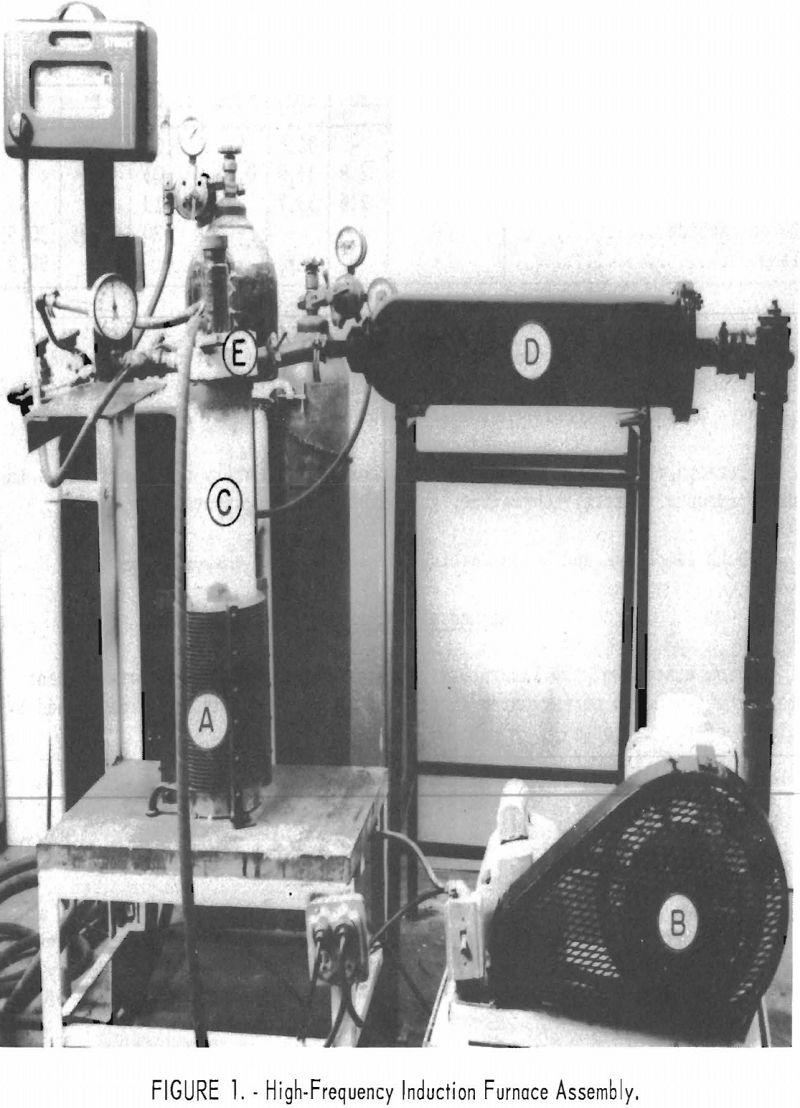
reaction. The filter chamber, D, placed between the silica-tube vacuum chamber and the mechanical pump to remove fumes and dust, is a converted high-pressure gas tank filled with stainless steel wool.
Pressure in the system is measured by a portable McLeod vacuum gage with a range of 1 to 5,000 microns.
Temperatures are measured with an optical pyrometer.
Materials
The composition of starting materials is shown in table 1.
Zircon “A” is ceramic-grade material, 100 percent minus 325-mesh. Zircons “B” and “C” are commercial-grade products from Florida beach sands, 100 percent through 80-mesh on 100-mesh. Zircon “B” consists of water-rounded grains. Zircon “C” consists of sharp natural crystals.
Foreign minerals present in the zircons are primarily other silicates in minor amounts (quartz, tourmaline, garnet, topaz, and staurolite).
Both lampblack and boron carbide are 100 percent through 325-mesh.
Method of Preparation
From a study of the literature and previous experiments it was evident that the synthesis of zirconium diboride from zircon might be accomplished by either of two methods:
ZrO2·SiO2 + B2O3 + 6C → ZrB2 + SiO + 6CO……………………………………..(1)
2ZrO2·SiO2 + B4C + 5C → 2ZrB2 + 2SiO + 6CO…………………………………(2)
Although a synthesis based on equation (1) obviously represents the most economical procedure, it was found impossible to reduce the carbon content of the product below 1 percent by this method. Our efforts were accordingly directed to synthesis of the material with boron carbide as the source of boron.
The zircon sands were digested overnight in 10 percent hydrochloric acid to remove iron, washed in water until acid-free, and fired at 150° C. until free from moisture.
Commercial boron carbide was found to contain approximately 1 percent of iron. This iron content was reduced by a hydrochloric acid leach to 0.14 percent. Free carbon, present in the boron carbide to the amount of approximately 8 percent, was allowed for in figuring the charge.
A typical charge consisted of zircon 76 percent, boron carbide 13 percent, and lampblack 11 percent. To this charge was added enough 10 percent solution of camphor in petroleum ether to moisten it; the charge was ballmilled with rubber balls for 2 hours. The blended charge was pressed into compacts at 5,000 pounds per square inch to reduce dusting and dried overnight at 140° C. to remove petroleum ether. Each charge weighed approximately 150 grams.
The blended charge was placed in the induction furnace, the furnace was pumped down to 50 microns, and heat was applied gradually for 1 hour to 800° C. to remove occluded and adsorbed gases.
To determine optimum conditions for the synthesis, charges were heated at temperatures ranging from 1,200° C. to 1,700° C., under a starting vacuum of 50 microns and for periods of 10 minutes to 12 hours.
The temperature at which reaction started was obtained by reading the optical pyrometer at the instant at which the first rise in pressure occurred. This primary rise in pressure was caused by the liberation of carbon monoxide or a mixture of carbon monoxide and carbon dioxide and invariably occurred at 1,350° ± 25° C. It was found impossible to record secondary reactions by tensiometric measurement because, once started, the reaction continued even when the application of heat was discontinued.
Experimental Results
The reaction product was soft, granular, and dark gray in color. Occasionally the bottom portions, which had been exposed to a somewhat higher temperature, appeared to be shiny and metallic and showed signs of incipient sintering.
The composition of the reaction products was determined both by X-ray and by chemical analysis. X-ray analysis showed, in those products in which the reaction was carried to completion, 95 percent or better zirconium diboride, with a total absence of silicates. In the chemical analyses boron was determined by fusing with sodium carbonate and titrating the neutralized solution with 0.1 normal sodium hydroxide in the presence of mannitol.
The zirconium content was determined by a combination of acid treatments followed by sodium carbonate fusion. The zircon was then precipitated with cupferron, ignited, and weighed as the oxide.
Silicon was determined by sodium peroxide fusion followed by dehydration with hydrochloric acid.
Total carbon was determined by the direct combustion-volumetric method.
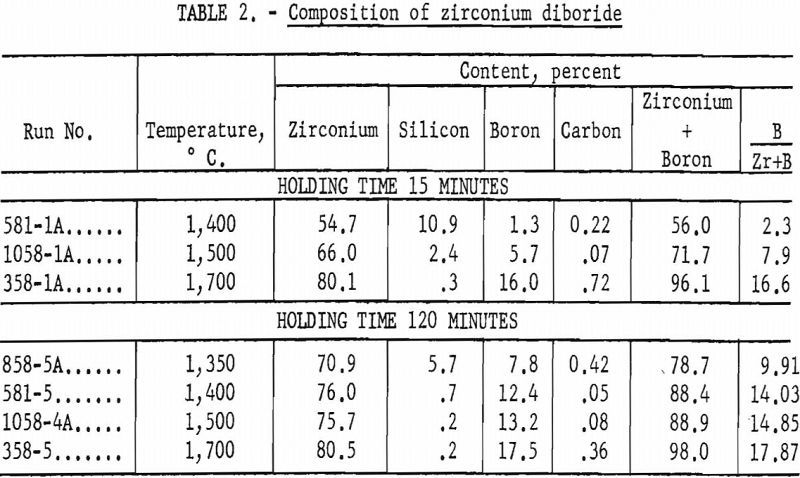
Chemical analyses of typical runs are shown in table 2.
Because these experiments were conducted under vacuum the nitrogen content of the final product was low; the average of nine runs showed 0.24 percent. The carbon content was within the same range; the average of eight runs showed 0.26 percent carbon.
The solubility of zirconium diboride, produced by this method in plus 100-mesh size and containing 78.4 percent zirconium and 16.6 percent boron is as follows: Hydrochloric acid, 59 percent; aqua regia, 96 percent; sulfuric acid, 100 percent; and hydrofluoric acid, 100 percent.
The density as determined in toluene at 25° C. was 5.97. Temperatures above the initial reaction temperature (1,350° C.) were considerably more efficient in removing silica and oxygen, and the products of these higher temperatures more nearly corresponded to the theoretical composition of zirconium diboride (table 2).
Run No. 358-5 at a holding time of 120 minutes gave a product that might be considered satisfactory for zirconium + boron production (table 2). Further experiments showed that at 1,700° C. and a holding time of 30 minutes the product contained 79.9 percent zirconium, 17.1 percent boron, 0.28 percent silicon, 0.54 percent carbon, and 97.0 percent zirconium + boron. Apparently at 1,700° C. the holding time may be reduced from 120 minutes to 30 minutes without greatly lowering the quality of the product.
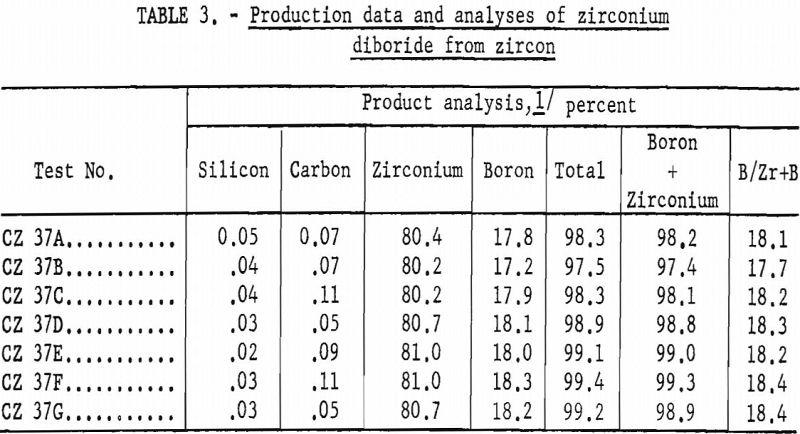
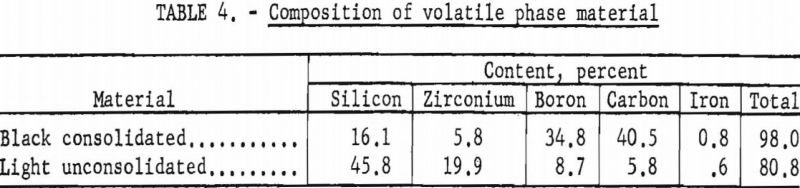
Seven experiments determined reproducibility of results (table 3). Volatile constituents, collected in a filter, showed two phases. One was a dense shiny black mass; the other was a light, unconsolidated, cream to light-brown material. Analyses of these two phases are shown in table 4. X-ray analysis of these volatile phases showed that the black product was mainly cubic silicon carbide and that the light material was chiefly silicon monoxide.
Discussion of Results
A typical analysis of commercial zirconium diboride shows 74 to 78 percent zirconium, 16.5 to 19.0 percent boron, and a 91 to 97 percent zirconium + boron content. Theoretical composition of zirconium diboride is 80.83 percent zirconium and 19.17 percent boron.
A holding time of 120 minutes at a temperature of 1,700° C. gave a product having an analysis comparable to that of a commercial product (table 2). From these experiments it is evident that a commercial grade of zirconium diboride may be made directly from zircon.
The measured density of 5.97 compares well with that obtained by Baroch and Evans (5. 96) for zirconium diboride prepared from the oxide and is somewhat higher than that reported by McKenna (5.64) for the diboride prepared from zirconium oxide, carbon, and boric oxide. The density, as determined from the lattice structure by Norton is given as 6.09.
A striking feature of this reaction was that the zircon was converted to zirconium diboride pseudomorphically; no change in particle shape was noted, even though the central portions of each grain were chemically completely converted. This feature is shown by the several panels in figure 2.