Table of Contents
Early studies in the carbonate leaching of uranium ores led to the investigation of pressure leaching as a method to enhance the dissolution of the metal values in this system. The initial investigations conducted at the University of British Columbia supplemented by the work done by the group at the A.E.C. Grand Junction pilot plant, all established the higher efficiency and better economics of the alkaline pressure leaching system. This work was conducted either in laboratory autoclaves using oxygen overpressures, or in pilot plant sized horizontal autoclaves with air as an oxidant.
Mill Description
Two distinct types of ore are treated at the mill, those occurring in the Westwater formation, and those occurring in what is known as the Poison Canyon formation, which incidentally is a somewhat refractory ore. It will suffice to say that the leaching circuits are fed a slurry containing from 12 to 15 percent plus-65-mesh material and from 35 to 50 percent of the material in the minus-200-mesh sizes.
The leaching section of the mill consists of two parallel circuits of eight series connected digesters, preceded by a preheat tank. The preheat tank is an insulated cylindrical tank of approximately 17,000 gallons capacity agitated by a 68 rpm Lightnin mixer equipped with a six-blade radial turbine impeller. The preheat tank raises the temperature of the primary thickener underflow from 95°F. to 150°F. The slurry is fed from the preheat tank into the first digester by means of a Gardner-Denver positive displacement pump. Each digester is a 12-foot-diameter, 12-foot-high vertical autoclave equipped with ellipsoidal heads, and designed to operate at 100 psig and 250°F.

Air Oxidation Studies
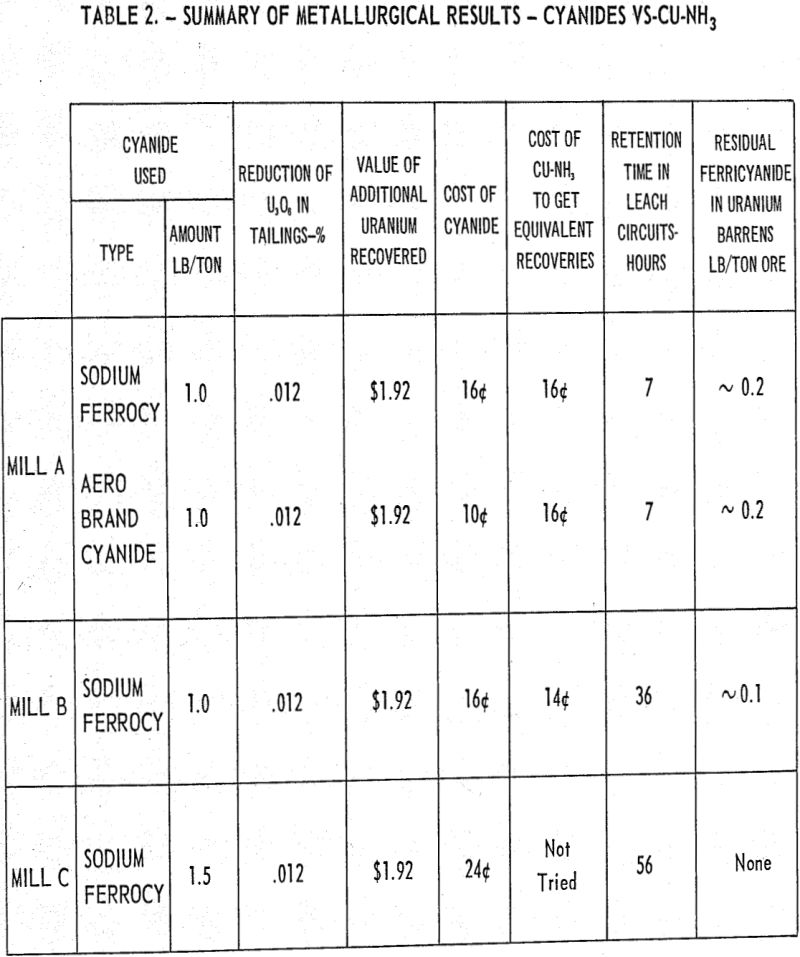
Adequate baffling is a must for gas dispersion in a liquid system. In addition to baffling, the factors such as peripheral speed of the impeller, the rotational speed of the impellers (rpm), and method of air injection all play an important part in the dispersion of air. Power requirements and shaft stability which are design considerations are affected by impeller diameters, rpm, and baffling and with a fixed design it is imperative to optimize these variables.
The data presented graphically ware obtained by batch testing at comparable air addition rates and leaching temperatures, and indicates that very little benefit was obtained by increasing the horsepower requirements by approximately 40 percent from the near optimum value of 0.54 hp/ton ore. It should be noted that the extraction value is the amount of U3O8 extraction obtained from the time the aeration was started, which was taken as zero time.
A consequence of these studies was the observation that the oxygen percentage in the offgases and consequently the oxygen partial pressure varied materially during the leaching period. The highest consumption of oxygen is occurring during the period covering ½ to 2 hours and tailing off to a minimum over the last 3 hours of the 8-hour leaching period.
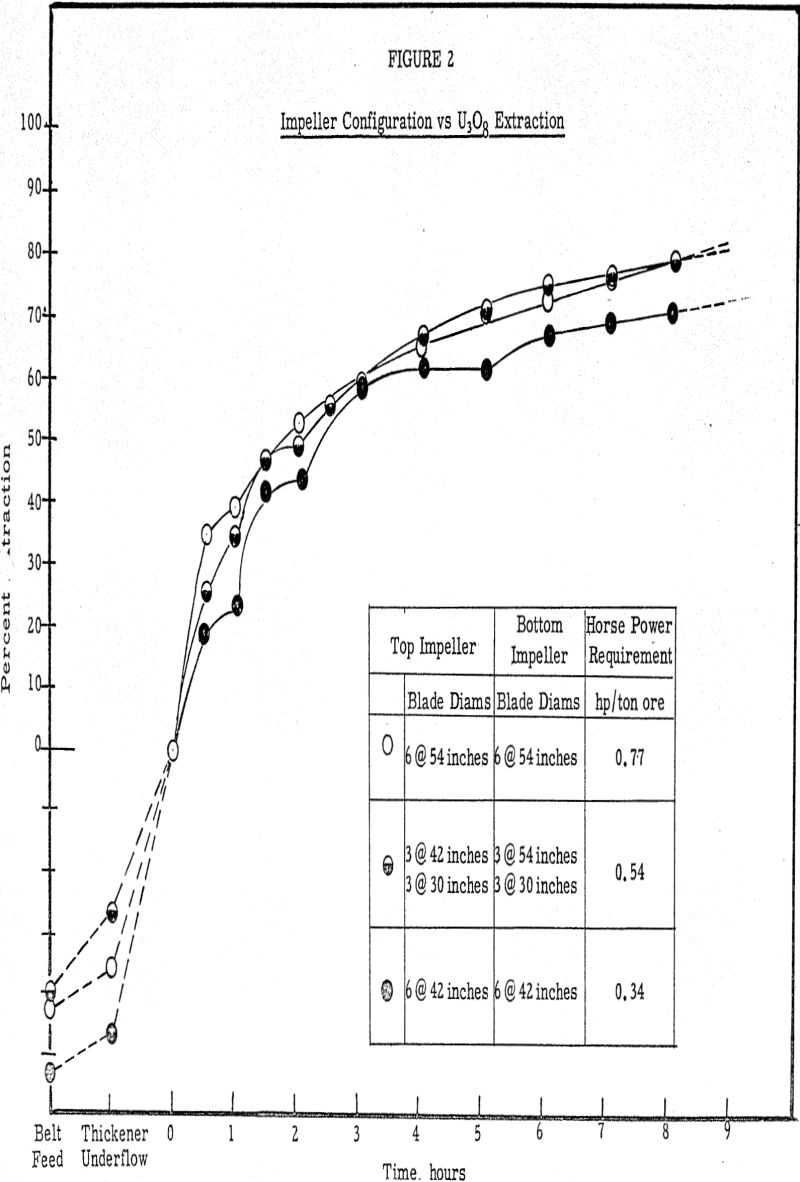
Laboratory testing conducted to supplement the inplant work substantiated the data that the critical period in the leaching process is the neighborhood of the first two hours. Laboratory tests conducted at excessively high air addition rates indicated that the uranium extraction suffered very little if the air was added for the initial two-hour period only and none for the last 6 hours, as compared with adding the air at the same rate over an 8-hour period
Study of Chemical Oxidants
The results of the air oxidation studies prompted the use of chemical oxidants in order to improve the extraction by the 4 to 6 percent margin, which was consistently obtained in the laboratory and not in the plant.
Potassium Permanganate – For the system tested, the use of permanganate and air did not improve the uranium extraction of that obtained with air alone.
Copper-Ammonia Complex – The use of copper-ammonia in the range of 2 pounds of copper sulfate and 8 pounds of NH3 per ton of ore did not enhance the extraction of uranium for the ores tested.
Cobalt and Nickel-Ammonia Complexes – The testing of these reagents is in the preliminary stages; however, the initial laboratory results indicate that these reagents provide greater oxidation than those others tested.
Calcium Cyanide and Sodium Ferrocyanide – Preliminary work with Cyanamid’s AERO Brand cyanide indicated that in the carbonate circuit, it was readily converted in the grinding circuit to the ferricyanide and that the ferricyanide was effective as a catalytic-type oxidation reagent. (The chemistry of this conversion is discussed fully later).
The scheme for testing the effect of the sodium ferrocyanide, which will be referred to as YPS (yellow prussiate of soda) was similar to that used for the crude cyanide. As shown in Figure 7, the optimum addition was well established to be at 1.0 pounds of YPS per ton of ore. No additional benefits were evidenced at higher concentrations. The YPS was added to the ball mill in all tests.
Oxidation-Reduction Theory for Alkaline Pulps
Most alkaline pulps contain a complex mixture of oxidizing and reducing agents and efficient extraction of uranium depends upon bringing this complex system into balance such that the final pulp will have a redox potential sufficiently high to oxidize all of the lower oxide forms of uranium to the hexavalent state and maintain them in that condition until the pregnant liquor can be filtered from the leach residue.
Factors working in favor of dissolution of the lower oxides of uranium are;
- An adequate supply of air and chemical oxidants
- Proper alkalinity and carbonate-bicarbonate ratio
- High temperatures and moderate pressures
- Fine grinding
- Good agitation and air dispersion
- Sufficient retention time in the leaching circuit
- Presence of catalytic elements, such as copper, ferric ion and vanadium.
Factors working against dissolution of uranium are:
- The presence of ferrous salts derived from pulp reactions with grinding balls, iron minerals in the ore, and the steel processing equipment
- The presence of reducing organic matter
- The presence of clays which produce thixotropic pulps which are difficult to agitate and aerate
- The presence of sulfides
- Poor air distribution
- Poor baffling and short circuiting in the leaching tanks
- Coarse grinding.
Results Obtained in First Plant Tests
Three mills in the Grants’ area pioneered the evaluation of cyanides as oxidation catalysts in alkaline circuits. All three used sodium ferrocyanide (Yellow Prussiate of Soda) added to the head of the leaching circuit in their first trials and two mills tested AERO Brand Cyanide added to the ball mill. The duration of the test periods ranged from ten days to several weeks. The quantities used ranged from 1 to 2 pounds of cyanide per ton of ore.
In all mills, the fact was confirmed that ferrocyanide can be oxidized to ferricyanide by air in full scale alkaline leaching equipment. The test with AERO Brand Cyanide proved that crude calcium cyanide in amounts of one pound per ton of ore can be oxidized quantitatively to ferricyanide in a plant ball mill within normal retention times with residual free cyanide in the mill discharge being less than could be detected by the standard silver nitrate titration.