Table of Contents
The heap leach cyanidation carbon adsorption electrowinning process developed has proved to be an economical method for exploiting low-grade gold ores and small isolated deposits not suitable for treatment by conventional cyanidation procedures. Heap leaching may be the most profitable method for processing selected gold and silver ores that do not require fine grinding and are readily amenable to cyanidation.
Gold mineralization is widespread in the United States. In 1967 the Bureau of Mines conducted an engineering appraisal of more than 1,300 lode and placer deposits, representing almost all of the Nation’s known gold reserves, to determine their gold product potential. These deposits were estimated to contain over 400 million ounces of gold; however, only 2 pct of this amount was found to be exploitable at $35 per ounce. A 400-pct increase in the price of gold within a period of 1 or 2 years caused a remarkable reevaluation of the type of material that constitutes ore. Low-grade materials containing as little as 0.03 ounce of gold per ton, providing sufficient tonnage is available, are now being field tested for precious-metal recovery. The Bureau of Mines and several mining companies have conducted extensive hydrometallurgical studies to exploit low-grade ores and mine waste material. The innovations developed include heap-leach cyanidation followed by precious-metal recovery from the resultant solutions by carbon adsorption or by precipitation on zinc, and new procedures for more efficient stripping of gold from activated carbon and separate recovery of gold and associated silver from enriched strip solutions. The purpose of this report is to review the state-of-the-art of heap-leach processing of precious metal ores and the application of granular activated charcoal for precious-metal recovery from cyanide solutions.
Heap leaching may be defined as the percolation leaching of piles of low-grade ores or mine waste that have been stacked or piled on specially prepared watertight drainage pads for pregnant liquor collection. This processing concept dates back to about 1752 when the Spanish miners percolated acid solutions through large heaps of oxide copper ore on the banks of the Rio Tinto River. Since then, this process has been used extensively throughout the world to leach oxide copper values from mine strip material from open pit mining of porphyry copper deposits. Uranium producers have also used heap leaching since the late 1950’s for extracting uranium from sub-mill-grade ores and for exploiting relatively small low-grade ore deposits. Heap-leach cyanidation is a comparatively recent development suggested by Bureau of Mines metallurgists in 1967 as a potential low-capital means for processing limestone and dolomite siltstone-type ores containing submicrometer particles of gold in a porous host rock.
Chemistry of Cyanidation
The basic principle of the cyanidation process is that weak alkaline cyanide solutions have a preferential dissolving action on the gold and silver contained in an ore. The reaction (Eisner’s equation) generally accepted for several decades as representing the dissolution of gold by cyanide solution is
4Au + 8CN- + O2 + 2H2O → 4Au(CN)2- + 4 OH-…………………………………..(1)
Recent research on the mechanism of cyanidation, however, indicates this reaction proceeds in two stages. Most of the gold dissolves by the reaction
2Au + 4CN- + O2 + 2H2O → 2Au(CN)2- + H2O2 + 2 OH-……………………………(2)
and a small but significant proportion dissolves via reaction 1. The gold dissolution rate is dependent on the concentration of NaCN and the alkalinity of the solution, the optimum pH being 10.3. For efficient leaching, the gold should occur as free, fine-size, clean particles in an ore that contains no “cyanicides” or impurities that might destroy cyanide or otherwise inhibit the dissolution reaction. An adequate supply of dissolved oxygen must be present in the cyanide solution throughout the reaction period.
The chemistry involved in the dissolution of gold in the heap-leach cyanidation treatment is the same as that for the agitation-cyanidation process. In heap leaching, the oxygen, essential for the dissolution of gold, is introduced into the cyanide solution as it is being sprinkled upon the ore heap. The adsorbed oxygen and carbon dioxide from the air may also cause chemical losses of cyanide according to the following reactions:
2NaCN + O2 + 4H2O → Na2CO3 + (NH4)2CO3………………………………………(3)
NaCN + CO2 + H2O → HCN + NaHCO3…………………………………………………(4)
In heap leaching highly oxidized ores, the decomposition of cyanide by carbon dioxide may be as great as that caused by the acid constituents of the ore. The decomposition of cyanide by carbon dioxide, as well as by ground acids, is minimized by using sufficient alkali such as lime (CaO) or caustic soda (NaOH) in the leach solution to maintain the alkalinity at a pH range of 9 to 11.
The mineral constituents of the ore and other foreign substances can influence the cyanidation process in a number of different ways. Silver normally occurs with gold either as an impurity in the particles of native gold or as silver-bearing minerals. The occurrence of silver in gold ores may range from less than 1 ppm to several hundred times the level of the gold present in an ore. Generally, silver is also dissolved by the cyanide solution and follows the gold through the process sequence. Iron sulfide minerals, which are common constituents of gold ores, are oxidized to some extent during the cyanide leach, thus resulting in the formation of acid. These acids are neutralized by the lime used in the cyanide leach sequence. Copper minerals may be dissolved by the cyanide leach solution and thus consume large quantities of NaCN and oxygen. Arsenic-bearing minerals may also interfere with cyanidation. Realgar (As2S2) and orpiment (As2S3) react rapidly with the cyanide solution and inhibit the dissolution of gold. Arsenopyrite (FeAsS), however, generally oxidizes very slowly in an aerated cyanide solution and has very little adverse effect on the leaching of gold. Stibnite (Sb2S3) strongly inhibits cyanidation. The presence of base metal ions such as Fe²+, Fe³+, Ni²+, Cu²+, Zn²+, and Mn²+ in the cyanide leach solution will retard the cyanidation of gold. In some cases the naturally occurring carbonaceous materials in sedimentary-type gold ores act as adsorbents for the gold dissolved by cyanide solutions. Charred mine timbers have similar properties and cause premature precipitation of gold. Organic substances such as decayed wood, oil, grease, and flotation reagents slow down cyanidation of gold by consuming the dissolved oxygen in the leach solution and also inhibits subsequent gold recovery from leach solution by precipitation of the gold on zinc dust.
Generally, gold and silver are recovered from pregnant cyanide solutions either by precipitation on zinc dust or by adsorption on activated carbon. For precipitation of gold on zinc, clarification of the pregnant solution is required to eliminate the suspended clayey constituents that can coat the zinc particles and retard precipitation of the precious metals. Elimination of the dissolved oxygen from the pregnant solution is essential to prevent the redissolution of the precipitated gold via reaction 1 or 2, and excessive zinc consumption through its interaction with the oxygen remaining in solution. The precipitation of gold on zinc is greatly improved by adding soluble lead salts, such as lead acetate or lead nitrate, to cyanide solutions to form a zinc-lead couple of greater activity. The reaction for precipitation of gold by zinc may be represented by the following equation:
NaAu(CN)2 + 2NaCN + Zn + H2O ↔ Na2Zn(CN)4 + Au + ½H2 + NaOH……………………(5)
Activated carbon has the capability of adsorbing the gold cyanide complex from cyanidation reaction pulps and unclarified cyanide effluents, thereby eliminating the liquid-solids separation, clarification, and deaeration processing steps that are employed in an all slime countercurrent decantation-zinc precipitation plant. Although activated carbon has been used in gold-silver recovery from cyanide solutions for several decades, the mechanism of gold adsorption on activated carbon is still not fully understood. One theory is that the gold is adsorbed as the gold cyanide complex Au(CN)2- and that this adsorption takes place by ion exchange. Some facts that support this theory are (1) CN- ions are also adsorbed by the activated carbon, (2) adsorption of Au(CN)2- ion is not accompanied by adsorption of an equivalent quantity of Ca²+ or Na+ ions from solution, and (3) the adsorbed gold can be desorbed or displaced with OH- ions provided by hot alkaline solutions. A recent study indicated that the gold adsorbed onto activated carbon is either in the calcium aurocyanide or hydrogen aurocyanide form, depending on the pH of the cyanide solution and the concentration and character of the “spectator” cations present.
Mineralogy of Gold Ores
The manner of gold occurrence and its association with the gangue minerals dictate whether or not the ore can be processed by the cyanide heap-leach method. From a metallurgical standpoint, gold ores may be roughly classified as (1) simple oxide ores containing fine particles of native gold in a quartz or limestone gangue, (2) simple sulfide ores in which the gold is associated with minor amounts of pyrite or arsenopyrite, (3) placer or alluvium material, (4) complex or refractory ores in which the gold-bearing mineral species are not readily soluble in cyanide solution, (5) complex base metal ores in which the precious metals are important economic constituents, and (6) base metal ores in which the precious metals are of minor value and are byproducts of treatment. Of these, only the simple oxide and sulfide ores and certain placers are suitable for heap leaching. These materials must possess the following characteristics: (1) Gold and silver values are leachable by cyanidation, (2) size of the gold particles is extremely small, (3) the host rock is porous to cyanide solution, and remains permeable during the relatively long leach cycle, (4) gold particles in ores of low porosity are liberated or exposed by fracturing and crushing, (5) the ore is free of carbonaceous material which has the capability of adsorbing gold cyanide and causes premature precipitation of the gold, (6) the ore is relatively free of “cyanicides” or substances that destroy cyanide or interfere with the gold-cyanidation reaction, such as partially oxidized sulfides of antimony, zinc, iron, copper, and arsenic containing minerals, (7) the ore does not contain excessive-amounts of “fines” or clayey constituents that will impede solution percolation, and (8) the ore is relatively free of acid-forming constituents that cause high lime consumption.
Types of gold-bearing deposits found amenable to heap leaching are as follows: (1) Limey siltstone containing submicrometer-size gold particles and minute amounts of pyrite, galena, cinnabar, and stibnite, (2) sillcified siltstones containing micrometer-size particles of gold, often associated with residual iron oxides, (3) sanded dolomite ore in which fine gold particles occur on the intergranular surfaces, (4) vein quartz ore in which gold occurs in ilmonite-rich cavities and fractures, (5) igneous host material cut with small quartz veins containing free gold and minor amounts of pyrite, (6) a schist containing free gold in the lamination of the rocks, and (7) siliceous quartz sinter of hot-spring origin.
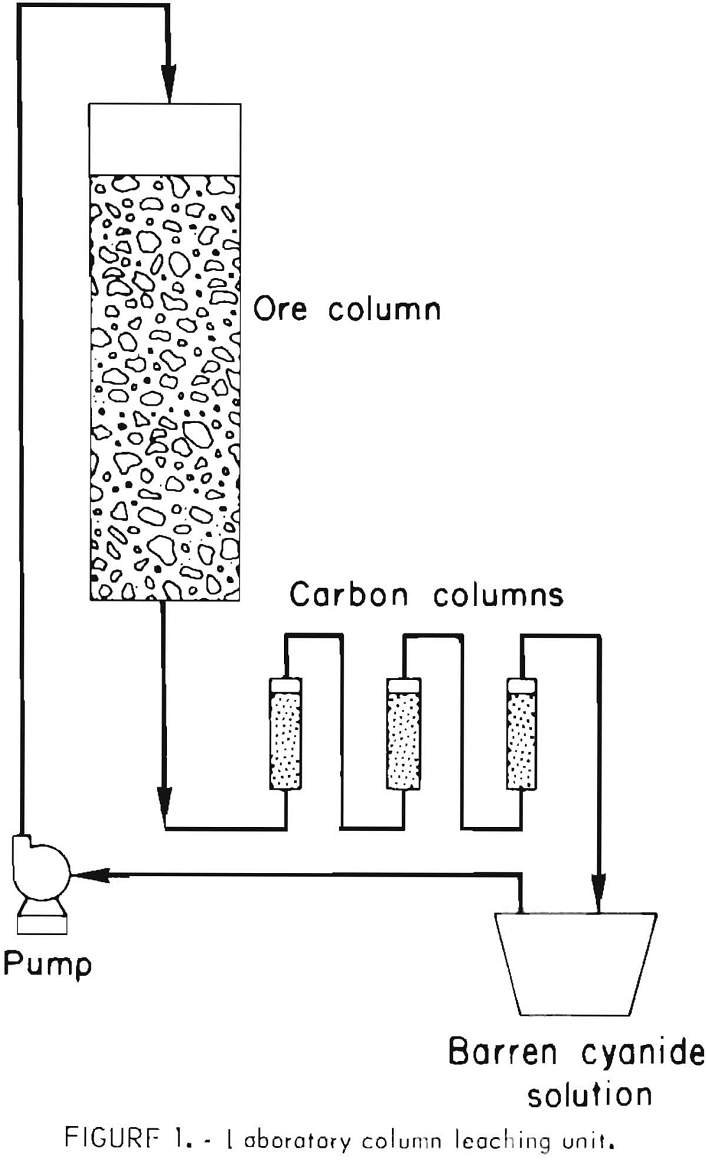
Gold Leaching Amenability Testing
Even though the mineralogical studies may indicate that the gold mineralization is associated with host rocks that are suitable for heap leaching, it is prudent to conduct laboratory and pilot tests for verification. The technology for determining whether or not a gold ore is amenable to percolation leaching with weak cyanide solutions is well established. Initially, bottle cyanidation tests are conducted on 200- to 500-gram charges of ground ore to determine the degree of gold extraction and reagent consumption. If the gold content of the ore is leachable at a relatively coarse grind, column percolation tests are made on ore crushed to various sizes, ranging from minus 2- to minus ¼-inch feed. The test apparatus is shown in figure 1. About 60 pounds of crushed ore is mixed with sufficient lime to provide protective alkalinity during cyanidation. This ore-lime mixture is transferred to a column about 6 inches in diameter to make a bed about 4 feet high. Twelve liters of solution containing 1.0 pound NaCN per ton is pumped to the top of the column, and the flow rate is controlled so that the solution trickles slowly downward through the ore. The pregnant effluent from the column is then passed upward through three small columns containing granular activated carbon and placed in series. The barren solution from the adsorption columns is recycled for additional leaching after making any necessary adjustments in the cyanide and lime concentration. Leaching is continued until no further significant amount of gold is extracted from the ore. Pilot-scale heap-leach tests may be conducted on a tonnage scale if it is desirable to confirm results obtained in laboratory column-percolation tests, or to further quantify reagent requirements (fig. 2). The percolation rate of solution through the ore can best be measured by conducting tonnage-scale experiments in columns containing a bed of ore 15 to 20 feet high.

Cyanide Heap-Leach Operations
Cyanide heap leaching is a comparatively recent hydrometallurgical development for exploiting low-grade gold ores, mine waste material, or deposits too small to justify construction of milling facilities. Although the process is analogous to heap leaching of copper ores, it is still considered to be in the developmental stage because many process variables influencing solution percolation and dissolution of the gold are not fully understood. Each commercial installation has developed an operating technique for pad and heap preparation and leaching procedure that meets the requirements of the ore. The technique selected depends largely on the manner of occurrence of the gold, physical characteristics and mineral composition of the ore, and the scale of operation.
All commercial cyanide heap-leach operations are being conducted on material stacked on impermeable pads. Watertight pads or bases are required to collect the pregnant solutions and to eliminate the possibility of losing gold and sliver cyanide solutions to the ground and contaminating local streams and underground water resources. The types of materials used for constructing the impervious pads include (1) compacted tailings mixed with bentonite, (2) asphalt (blacktop) or lignin sulfonate mix placed on compacted gravel and covered with an asphalt sealer, (3) reinforced concrete pads, and (4) plastic or rubber sheeting laid on a smooth excavated area and covered with 2 to 3 feet of washed sand and gravel.
Basically, two methods of heap-leach cyanidation are used commercially: (1) short-term leaching of crushed ore, and (2) long-term leaching of run-of- mine material.
In practicing short-term, heap-leach cyanidation, the ore is crushed to a small size, stacked 4 to 8 feet high on permanent pads, each with a capacity ranging from 1,000 to 10,000 tons, and leached by sprinkling the top of the heap with dilute cyanide solution (fig. 3). Cyanide solution percolates through the heap dissolving the gold and silver values. It is subsequently collected on the watertight pad, which is sloped to permit the pregnant solution to flow into channels for transfer to a storage pond or tank. The feed material is crushed to a size that will give good liberation or exposure of the gold mineralization to cyanide solution and still obtain reasonable percolation rates. In typical operations, ores are crushed to minus three-fourths inch, and as fine as minus one-fourth inch in the case of a gold-quartz ore. The leach cycle is measured In days, generally from 7 to 30. When the leach cycle is completed, the waste is removed from the pad and a new batch of crushed ore is applied. The Carlin Gold Mining Co. started this type of leaching on mine cutoff material crushed to three-fourths inch in 1971. The Smoky Valley Mining Co. has completed the installation of a 8,000-ton/day short-term heap leach-carbon adsorption-electrowinning facility at Round Mountain that went on-stream December 1976.
Long-term leaching is used primarily to extract gold from uncrushed, porous, sub-mill-grade material from open pit operations. The ore charges are run-of-mine material generated by blasting, and may contain some large
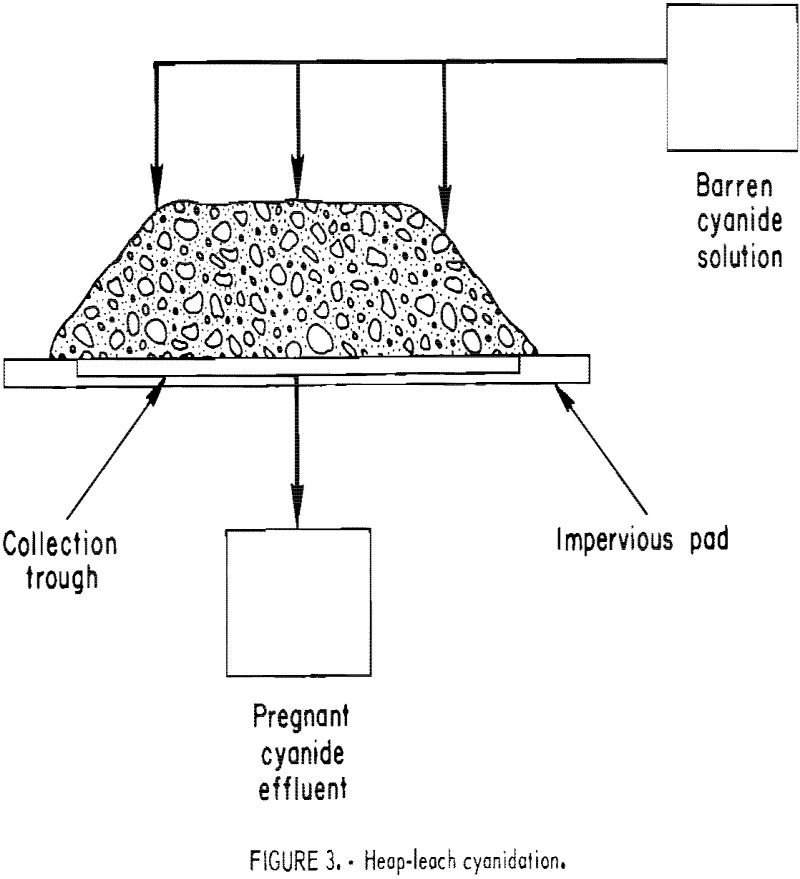
boulders, but most of the feed is minus 6 inches in size. The tonnage of the heaps under treatment usually ranges from 10,000 to 2,000,000 tons. Most leach heaps are shaped to resemble a truncated pyramid 20 to 30 feet high, as depicted in figure 3. The height is governed by factors such as ore permeability and the maintenance of protective alkalinity, cyanide strength, and sufficient dissolved oxygen in the leach solution as it percolates downward through the heap. The leach cycle is measured in months. These heaps are leached until it is no longer profitable to continue the operation, which may be for years. Upon termination of the leach, the residue is left on the pad. Cortez Gold Mines has completed heap leaching approximately 2 million tons of run-of-mine cutoff material. Cortez operated the first known integrated heap-leach cyanidation-carbon adsorption-electrowinning plant at its Gold Acres property, about 8 miles from the main cyanide plant.
Generally, the cyanide leach solutions are introduced onto the heaps by spraying from perforated plastic pipes, by sprinkling using plastic sprinkler heads, or by ponding. Typical rates of application of the leach solutions range from 5 to 75 gal/ft² of surface, area per day. Pond leaching, as illustrated in figure 4, was applied successfully by the Idaho Mining Corp. to extract the readily leachable gold values from a sanded dolomite ore that
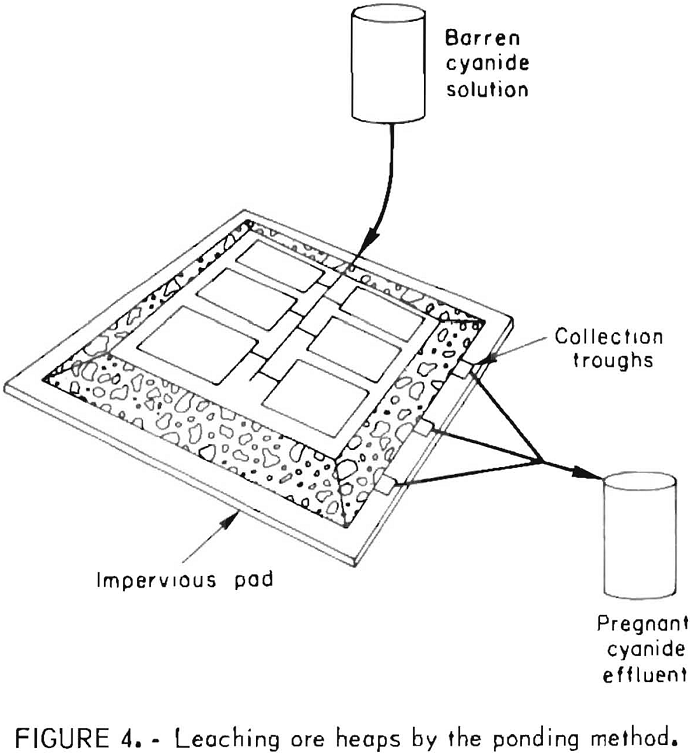
exhibits slow percolation characteristics. One hundred and sixty thousand tons of ore was stacked about 25 feet high and leveled; the flat surface of the pile was divided by small dikes into nine ponds to facilitate control of leaching solutions. The heap was washed with barren solutions from the carbon adsorption plant.
In commercial application, solution strengths range from 0.5 to 1.0 pound of sodium cyanide per ton of solution, and the pH is maintained at about 10 with the application of lime (CaO) or caustic soda (NaOH).
Gold-Silver Recovery From Process Solutions
Based on the availability of existing conventional cyanide mill facilities with a zinc precipitation circuit, the gold and silver contained in heap-leach pregnant solutions may be recovered from solutions by conventional Merrill-Crowe precipitation. If mill facilities are not available or if the concentration of gold in solution is below a nominal 0.05-oz/ton solution, the preferred method for recovering the precious metal values from heap-leach effluents is by adsorption on activated carbon. Not only is the carbon adsorption process more efficient than zinc precipitation for treating dilute gold-silver cyanide solutions, but the method also entails lower capital and operating costs.
The carbon adsorption method became practical on development of a usable method for stripping the precious metal values from the activated carbon so it could be recycled in the system. Today, the use of activated carbon in conjunction with heap leaching is a simple, economical process that is suitable for exploiting lean resources or small ore bodies. The capital investment for this process is estimated to be about 20 to 25 pct of the cost of a conventional countercurrent decantation cyanide plant, and operating costs are about 40 pct of a conventional cyanide plant.
In typical continuous carbon-adsorption operation, the gold cyanide effluents from heap leaching are pumped upward through from three to five columns or tanks in series which contain granular activated carbon. The harder carbons, manufactured from coconut shells, are preferred for use because there is less tendency for breakage or abrasion. The amount of gold and silver that can be loaded on the carbon will vary greatly, depending on the type of ore and the tenor of the cyanide leach solution. Some factors that influence the loading are (1) the concentration of gold and silver in cyanide leach solutions, (2) ratio of gold to silver, (3) pH of leach solution, (4) concentration of impurities, (5) flow rate, and (6) type and particle size of granular carbon employed. Typical loadings obtained commercially range from 200 to 800 ounces of gold, or combination of gold and silver, per ton of carbon. However, the adsorption phenomena is not restricted to gold and silver cyanide complexes. Activated carbon is capable of adsorbing a large variety of organic substances, and inorganic constituents such as silicic acid, and metallic ions such as calcium, copper, nickel, mercury, and iron, thus reducing the number of sites available for the precious metals. The preferred pH of the leach solution to obtain efficient gold adsorption ranges from 9 to 11. The concentration of free cyanide in the leach solution should be maintained at a value that is as low as possible and still be consistent with satisfactory metal extraction from the ore. Finer size carbon particles adsorb greater amounts of gold and at a faster rate than coarse carbons; however, the mesh size should not be so small that it results in particulate carbon losses to the process solutions during the adsorption step. The adsorptive capacity of activated carbon manufactured from coconut shells for gold cyanide is generally greater than that of carbons prepared from petroleum coke, wood, or coal. Also, higher concentrations of gold in pregnant cyanide solutions produces higher gold loading on the carbon. Similarly, higher gold loading on activated carbon and the production of lower gold-bearing barrens are achieved by using lower flow rates.
There are two methods for loading activated carbon. In one method, the gold-bearing cyanide solution is percolated downward through a fixed bed of activated carbon. In the other, the pregnant cyanide solution is pumped upward through the carbon at a velocity sufficient to maintain the bed of carbon in a fluidized state or suspended in the solution stream without being carried out of the system. The choice of loading technique depends on the amount of turbidity or slimes present in the heap-leach effluents. The fixed beds or packed carbon columns are limited to a maximum solution flow of about 5 gpm/ft² of cross-sectional area. The feed solutions must be free of particulate matter because the bed of carbon behaves as a sand-filter and will eventually become plugged if slimes are present. The only advantage of the fixed-bed carbon is that the amount of carbon required is less than that required for a fluidized system processing the same amount of solution.
The fluidized-bed adsorption system is generally used in commercial practice for adsorbing gold cyanide values from unclarified leach solutions containing minor amounts of slimes. Four important process variables to be considered in the design of a fluidized bed activated carbon loading system are (1) flow rate of the feed solution, (2) average daily production of precious metals, (3) maximum amount of gold that can be loaded on the carbon, and (4) the particle size of carbon employed. Item 1 is based on the volume of pregnant solution generated daily from the heap-leach operation. Item 2 may be based on a weighed maximum gold concentration in solutions obtained from several heaps placed in operation at staged intervals. The inventory of carbon required in the plant and the size of adsorption and stripping equipment can be minimized by utilizing the adsorptive capacity of the carbon for gold to the fullest extent that is practical. A loading of 400 ounces of precious metals per ton is considered desirable by the industry.
However, if the incoming solution contains less than 0.05 ounce of gold per ton, the loading usually will not exceed 200 oz/ton of carbon. The particle size of carbon used commercially in heap-leach cyanidation is either minus 6 plus 16 or minus 12 plus 30 mesh. Generally, the solution flow rate required to maintain fluidization in a bed of carbon containing minus 6- plus 16-mesh particles is about 25 gpm/ft² of cross-sectional area of the column, whereas for the minus 12- plus 30-mesh carbon, the required flow rate is 15 gpm/ft². Under these conditions, the carbon bed expands about 50 pct. The depth of the bed of carbon at rest should not be more than three times the diameter of the column. The height of the column should be 2.5 to 3 times the height of the carbon at rest to allow for proper expansion of the bed and to provide enough free board to allow for solution surges. The amount of carbon required for a fluidized-bed adsorption system employing minus 12- plus 30-mesh carbon particles is about 10 times the daily amount of carbon stripped. For the minus 6- plus 16- mesh carbon, the carbon inventory should be about 15 times the daily amount of carbon stripped because of higher solution velocity and shorter contact time. Industrial experience has shown that the carbon charge should be equally distributed through four or five columns or tanks in series for an efficient carbon adsorption system.
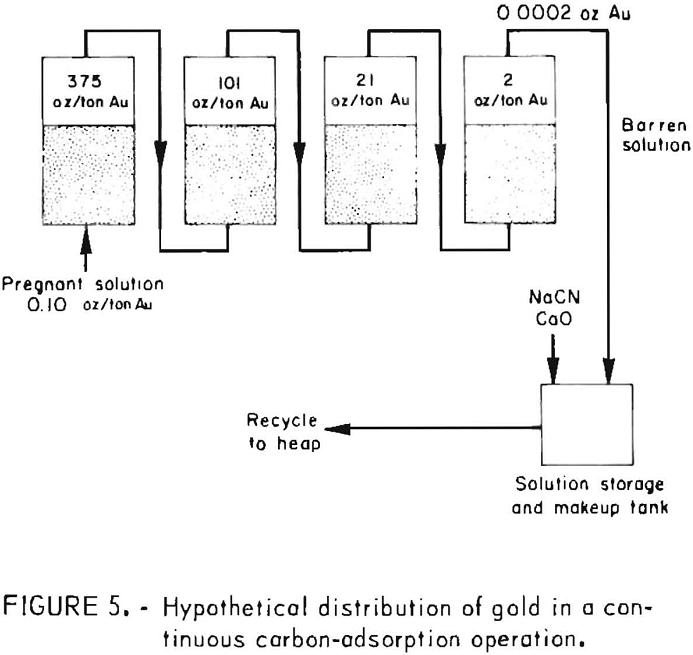
During startup of a countercurrent carbon adsorption system for processing heap-leach cyanide effluents, the first or lead column of carbon may adsorb all of the precious metal values. After the carbon has been in service for some time and carries about 20 ounces of gold per ton, a gradual break-through of gold will occur in the effluent from the first column and he adsorbed by the succeeding bed of carbon. As the carbon in the first column becomes loaded with more gold and its adsorptive capacity decreases, the gold content of the column effluent solution gradually increases. It is obvious from monitoring the progress of adsorption that to obtain efficient utilization of the carbon gold. It is advantageous to use several charges of carbon in series. Effluent samples should be collected at regular intervals and analyzed, preferably by the atomic absorption technique to obtain rapid gold and silver assays at the plant site. When the analyses indicate that the lead charge of carbon has adsorbed the desired amount of gold or becomes saturated, a portion of this carbon charge (representing the daily production of loaded carbon) is removed, and an equivalent amount from each of the other columns is advanced one column. An equivalent amount of fresh activated carbon is added to the last column to insure the production of barren solutions low in gold content for recycling to the heap. Figure 5 illustrates the hypothetical distribution of gold attainable in processing 5,000 tons of cyanide solution containing 0.10 ounce of gold per ton and employing four 1-ton beds of carbon in series.
The precious metal values presently are recovered from loaded carbon by two radically different techniques. In one practice, adopted by some small companies with limited capital for investment in the required equipment, the loaded carbon is shipped to smelters where the product is burned to recover a
dore bullion by smelting the ashed residue. Typical smelter charges including the nonpayment for 7.5 pct of the total gold content will average about $3,500 per dry ton of carbon containing 300 ounces of gold per ton. Additional costs to be considered are the purchase price of the activated carbon and haulage charges. Thus, a reasonable cost figure for processing a ton of loaded carbon by the smelting technique is $5,500 to $7,000. In the preferred method, gold recovery from activated carbon is accomplished by desorbing the gold and silver values from the carbon and electrowinning the values from the resultant strip solutions. This permits repeated reuse of the carbon, which greatly enhances the economics of the carbon cyanidation process. The first practical desorption process was developed by the Bureau of Mines in 1952. The process (commonly referred to as the Zadra process) employs a 1.0 pct NaOH-0.1 pct NaCN strip solution at 93° C and at atmospheric pressure to desorb the gold and silver from the carbon. The gold-silver desorption step, which is dependent upon the concentration of alkali (NaOH) and the temperature of the strip solution, is greatly enhanced by the addition of an alcohol such as ethanol or methanol to the desorbent or stripping solution. The precious metal values contained in the strip solution are electrowon from the solution, and the barren solution recycled for additional stripping. Current industrial application of the Zadra desorption process indicates that 50 to 100 hours are required to strip the carbon from a loading of 300 ounces of gold to less than 5 ounces of gold per ton of carbon. The Bureau at the Salt Lake City Metallurgy Research Center developed a pressure stripping method that greatly reduces stripping time and reagent consumption.
Economics of Heap-Leach Operations
Pizarro described in detail the 10,000-ton-per-month heap-leaching operation at the Carlin Cold Mine, Carlin, Nev. The heap-leach effluents join the pregnant solutions from the Carlin mill for clarification followed by conventional zinc dust precipitation for gold recovery. Reagent consumption was minimal, 0.1 pound NaCN and 1.0 pound CaO per ton of ore. Pizzaro and McQuiston presented several excellent tables of Carlin’s operating costs. Based on these data, the calculated heap-leaching operation costs at Carlin were $0.96 per ton of ore in 1973 and $1.21 per ton in 1974.
Mountain States Research and Development, Tucson, Ariz, made an economic evaluation on four processes commonly employed for recovering gold from low-grade ores. The extraction processes evaluated were (1) heap leaching with carbon adsorption, (2) vat leaching with carbon adsorption, (3) conventional countercurrent decantation, and (4) carbon-in-pulp cyanidation. Bhappu indicated that the heap-leach process is economically justified in the processing of lean ores averaging 0.04 ounce of recoverable gold per ton at gold prices of $80 per ounce for multimillion-ton ore bodies. For the smaller operations, labor costs would be proportionately much larger per ton, and the costs for equipment and facilities would differ widely depending on the accessibility of the mine.
Chisholm reported that a small mining company in New Mexico brought into production a 500-ton-per-day open pit gold mine and heap-leach operation at a total cost of $150,000. A breakdown of the costs for items of equipment purchased and for mine facilities was presented.
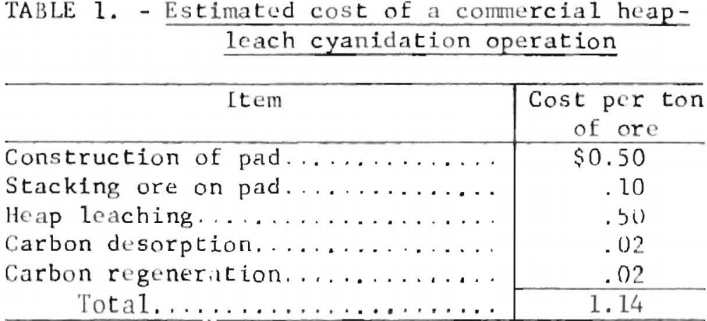
Additional cost factors that appear to substantiate the foregoing costs were developed as a result of information gathered from several sources. Based on a 500,000-ton heap-leach operation, typical costs for preparing an asphalt pad, including preparation of site, subbase, sealer, and covering the asphalt with a layer of gravel are $0.50 per square foot. This is equivalent to $0.50 per ton of ore, assuming that a ton of ore is stacked per square foot of pad surface. The stacking of ore on a pad reportedly costs about $0.10 per ton. The leaching costs are dependent on reagent consumption and length of period of treatment, and thus can vary appreciably. A reasonable leaching cost reportedly is $0.50 per ton of ore. Operation of the carbon desorption unit costs about $0.02 per ton of ore, assuming that the ore contains 0.05 ounce of recoverable gold per ton and that the carbon is loaded with 400 ounces of gold per ton. Prevailing practice is to regenerate the leached carbon before reuse by heating the carbon in a steam atmosphere at —700° C in a rotary kiln. Based on cost figures stated in a recent article on carbon regeneration, the cost for reactivating the stripped carbon is estimated to be $0.02 per ton of ore. A summary of the cost estimates, excluding mining costs, is shown in table 1.
Cyanide Handling and Disposal
The precious-metals mining industry for many years has promoted the health and safety of its employees regarding the handling and use of cyanide. The industry has demonstrated that, with proper training and instructions, cyanide can be used routinely in leaching gold-silver ores with little risk to the worker. However, growing concern about occupational hazards and environmental pollution has resulted in the promulgation of regulations that require industry to comply with standards and guidelines established by Federal, State, and county regulatory bodies. The Occupational Safety and Health Administration (OSHA) in October 1976 published its recommendations designed to protect the health of employees working with cyanide salts. The publication, “Criteria for a Recommended Standard-Occupational Exposure to Hydrogen Cyanide and Cyanide Salts [NaCN, KCN, and Ca(CN)2]” can be purchased from the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402.
Employees working at a heap-leach cyanidation installation may be exposed to cyanide in the form of dust and solutions, especially during mixing of concentrated stock cyanide solutions. Ingestion of as little as 0.20 gram of sodium cyanide is considered to be lethal for human beings. The heap-leach operation itself is considerably less hazardous because the leach is conducted in an open area with maximum ventilation. By maintaining the alkalinity of the leach solution at pH 10 to 11, the possibility of generating hydrogen cyanide gas (HCN) is minimized, and only trace amounts of HCN can be released by interaction of NaCN and CO2 in the environment. Measurements made by Mining Enforcement and Safety Administration (MESA) inspectors show that the HCN concentration in the air close to a working heap is consistently only 2 to 3 ppm. This is significantly less than the limit of 10 ppm established by OSHA for sustained breathing of gaseous cyanide.
In a well-designed heap-leach installation, the pregnant cyanide solution settling pond, which catches the drainage from the heap, should be designed so that the capacity of the pond is sufficient to accommodate the maximum rainfall and runoff that can be expected for that particular locality, thus preventing the discharge of cyanide solution to the watershed during operation and after abandonment of the leached ore heap. The settling ponds are generally earth fill structures that are lined with watertight polyvinyl chloride or polyethylene sheeting. Ponds holding cyanide solutions should be adequately posted and fenced to restrict access to the area.
Because of the appreciable evaporation losses that occur during heap leaching, most operators are able to maintain complete recycling of the leach and wash solutions. Thus the need to discharge potentially hazardous solutions to maintain the water balance for the leach operation is circumvented. If a bleedoff system is required, in the event of an abnormally heavy rainfall, cyanide removal techniques must be considered. The not widely used method for reducing free cyanide and heavy-metal cyanide concentrations in waste streams involves chemical treatment with chlorine or hypochlorite. The reaction mechanism is believed to be as follows:
NaCN + 2NaOH + Cl2 → NaCNO + 2NaCl + H2O…………………………………..(6)
2NaCNO + 4NaOH + 3Cl2 → 2CO2 + 6NaCl + N2 + 2H2O……………………..(7)
The available chlorine may be furnished as chlorine gas or a hypochlorite solution. Approximately 1 pound of calcium hypochlorite, Ca(OCl)2, will oxidize 1 pound of free cyanide. The term “heavy metals” generally denotes those metals that are of particular concern in cyanide treatment such as copper, silver, zinc, cadmium, mercury, arsenic, chromium, manganese, iron, and nickel. The main method employed for removal of the heavy metals after the destruction of cyanide is by addition of lime to precipitate the metals and to promote flocculation of the precipitates. The heavy-metal precipitates would be filtered and shipped to an approved toxic-substance landfill for disposal. The U.S. Environmental Protection Agency (EPA) regulations for limitations of cyanide discharge in waste solutions is 0.02 ppm CN.
It is clearly advantageous from an economic standpoint for the mine operator to lower the soluble gold losses in the heap to a minimum by thorough washing of the leached ore with fresh water. The washing step results in recovery of most of the dissolved gold and a large portion of other cyanides remaining in the heap as free cyanide or complexed with heavy metals. Heavy-metal cyanide salts are known to persist for several years, but residual free cyanide in abandoned heaps is believed to exist no more than 1 month, depending on climatic conditions; however, scientific data to support this contention are lacking. The retention and fate of residual cyanide in heap leached residues is being scrutinized to an increasing extent by regulatory agencies.
Abandoned heaps are less susceptible to wind and water erosion than finely ground tailings impounded behind a dam. In the semiarid regions of the Western United States, where most of the heap leach cyanidation is being practiced, invasion of the abandoned heaps by native desert flora has been observed to occur within 1 or 2 years.
Under provisions of the Federal Metal and Nonmetallic Mine Safety Act of 1966 (Public Law 89-577), MESA is responsible for the enforcement of the health and safety standards prescribed to protect the workers at all mine sites, which includes heap-leach cyanidation operations. With the signing of the Federal Mine Safety and Health Act of 1977 (Public Law 91-173), it became the responsibility of the Secretary of Health, Education, and Welfare and the Secretary of Labor to develop and promulgate improved health and safety standards for persons working at mining properties. New and/or revised regulations will be implemented by the Mining Safety and Health Administration (MSHA) under the Department of Labor. The effective date of these rules and regulations is expected to be March 9, 1978.
Under provisions of the Resource Conservation and Recovery Act of 1976 (Public Law 94-580), EPA is responsible for the development of regulations and guidelines for disposal and management of all solid wastes, including mining wastes. EPA currently is conducting a detailed study on the adverse effects on the environment of solid wastes from active and abandoned surface and underground mines. Guidelines for the disposal of mining wastes are expected following the completion of the study in June 1978. Meanwhile, State and/or county governments where heap-leach cyanidation operations exist regulate the disposal or abandonment of cyanided ore heaps.
Recent Innovations in Gold-Silver Recovery from Cyanide Process Solutions
Recently, efforts have been directed toward the development of lower cost gold-processing procedures that will improve the economics of treating lower grade ores and deposits too small to warrant construction of conventional mill facilities.
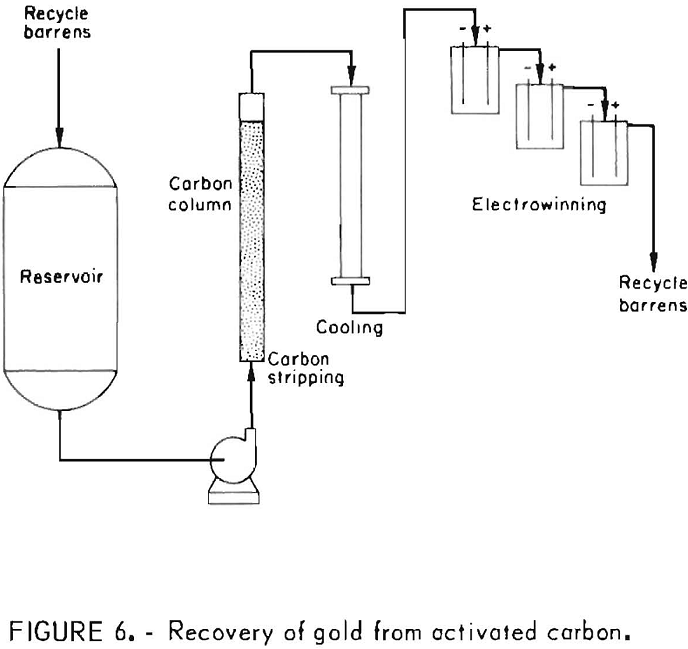
The Bureau of Mines developed a more efficient process for recovering gold and silver from laden activated carbon. Factors that can affect the performance of the modified strip solution include temperature, and concentrations of NaOH and ethanol. The strip solution, which is water containing 1 wt-pct NaOH and 20 vol-pct ethanol, is heated to 80° C and circulated through a bed of loaded carbon to desorb precious metals and cyanide ions. The precious metals arc recovered by electrolysis, and the depleted electrolyte is recycled to the carbon desorption column. Five to six hours of simultaneous stripping and electrowinning will desorb up to 99 pct of the precious metal values carried by the activated carbon. A flow diagram for the process is shown in figure 6.
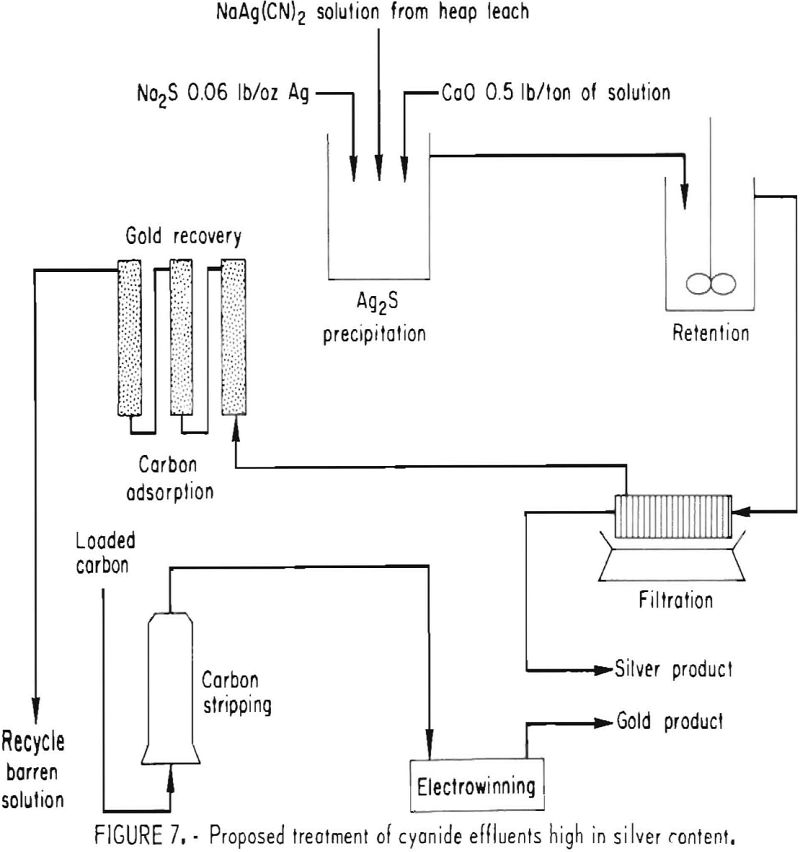
Activated carbon, which had been used for carbon-in-pulp cyanidation of gold ores for several years, is now playing an important role in the recovery of gold from heap-leach cyanidation effluents. However, application of this technology to the treatment of gold ores that are rich in silver greatly increases the amount of activated carbon needed to adsorb the equivalent dollar value of silver from an equal volume of leach solution. A processing sequence was developed that circumvents the handling of large quantities of activated carbon. It consists of recovering the silver selectively as a Ag2S precipitate using sodium sulfide, and after filtration, recovering the gold from the filtrate by carbon adsorption. The key to this process is the formation of readily filterable Ag2S flocs by agitating the slurry with 0.5 pound of CaO per ton of solution for about 1 hour. The process sequence is shown in figure 7. The Ag2S precipitation-carbon adsorption processing method is being evaluated at a heap-leach cyanidation installation by Sand Springs Co., Fallon, Nev., for processing a silver ore low in gold content.
The sodium sulfide precipitation procedure was also shown to be effective for selectively precipitating silver from highly concentrated gold-silver effluents obtained in the initial stages of stripping precious metal-laden activated carbon. The method provides a means for the subsequent production of a low-silver gold bullion. Silver is precipitated selectively from strip solution by the same procedure used previously for silver precipitation from pregnant cyanide leach solutions. Filtrates containing less than 1 ppm Ag and up to 800 ppm Au were obtained. The precipitates, which contain diatomaceous earth, lime, slimes, etc., as well as Ag2S, assay 3,000 to 6,000 ounces of silver per ton and represent 99-pct silver recovery. The entrained gold amounts to less than 0.1 pct of the total precious metal content. The
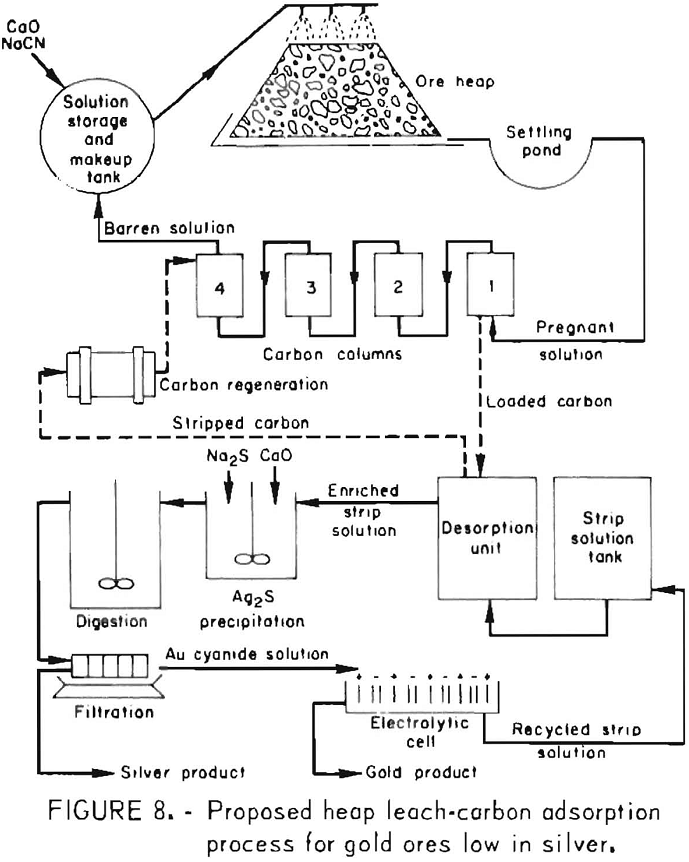
silver-free filtrate can then be electrolyzed to win the gold by deposition on steel wool cathodes. Barren electrolyte recycled to the desorption unit can be used to desorb more values from the carbon. Finally, the gold-laden steel wool cathodes when mixed with proper fluxes can be refined into gold bullion low in silver. Figure 8 shows a conceptual flow diagram depicting the heap leach-carbon adsorption-desorption system and the precious metals separation and recovery cycle.
