Table of Contents
Three fundamental methods are employed in decomposing phosphate rock:
- Reduction by carbon at high temperature.
- Treatment with one or more acids.
- Calcination or fusion with silica, sodium salts, magnesium minerals, or small quantities of phosphoric acid.
The products derived from phosphate rock by these treatment methods may be divided into the following live broad groups:
- Elemental phosphorus.
- Phosphoric acid.
- Phosphatic fertilizers.
- Phosphate salts.
- Other phosphorus compounds.
Phosphorus and phosphoric acid have certain direct industrial applications, but they are more commonly used as intermediates in manufacturing the products included in the other three groups.
The various intermediate and end products derived from phosphate rock and their more important industrial applications are shown in figure 1.
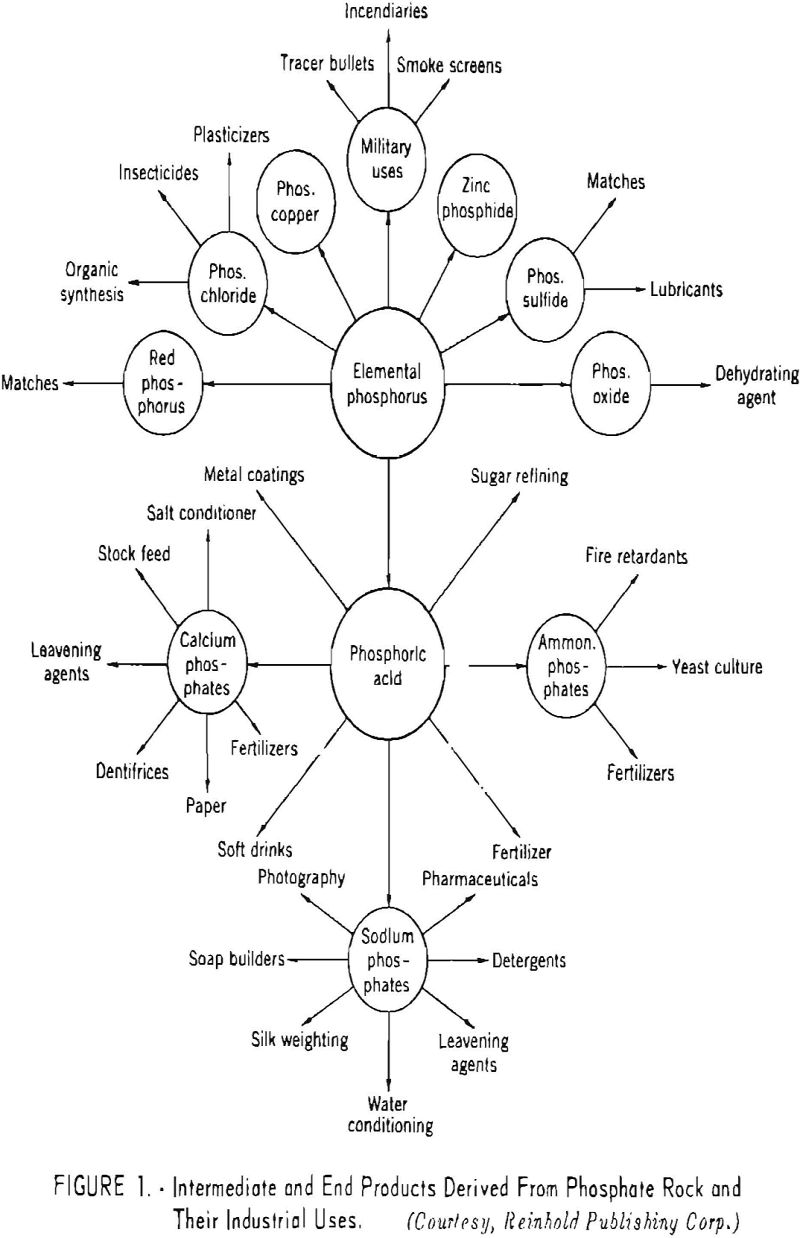
The earliest use of phosphatic materials was for fertilizer purposes, but fur many years these materials were applied in a raw or natural state.
The dung of birds was employed by the Carthaginians before 200 B.C., and the early agricultural writers recommended applications of natural phosphate materials to increase crop yields . The Incas of Peru and the American Indians applied fish and guano to the soil long before America was discovered by Europeans. Although the composition of such phosphate materials was unknown, their function in increasing crop yields was generally recognized. In 1769 Gahn, a Swedish chemist, reported that phosphorus was an essential constituent of bones, but the untreated natural materials continued to be the main sources of fertilizer until about the middle of the 19th century.
In 1840, Leibig suggested dissolving bones with sulfuric acid to render the contained phosphorus soluble and available to crops. In 1857 he pointed out that phosphate rock could be treated in a similar manner. This marked the beginning of a commercial fertilizer industry, which has grown to enormous proportions and now includes many manufactured products derived from phosphate rock.
Elemental phosphorus was first produced in 1669 by Brandt, a German chemist, who on treating desiccated urine in a retort found that a vapor was evolved that burst into flame on contact with air, forming dense, white fumes. He called this vapor phosphorus or “light bearer.” Later, it was found that this vapor could be condensed to a yellow liquid (if protected from air) and collected under water.
Elemental phosphorus was manufactured on a small scale by reducing acidulated bones or phosphate rock with carbonaceous materials, but 200 years elapsed before Readman (1889) patented the first practical electric furnace for making elemental phosphorus.
The commercial production of phosphorus in America was undertaken by the Oldbury Electrochemical Co. in 1896, using a relatively small single-phase furnace with an annual capacity of about 750 tons of phosphorus.
For many years the demand for phosphorus was small, but during World War I manufacturing facilities were expanded rapidly to supply military requirements. After World War I the phosphorus industry continued to grow, owing to improved processing and recovery methods and to increased demand for high-quality phosphate products. Elemental phosphorus requirements during World War II, coupled with the availability of relatively low cost, hydroelectric power, caused further expansion. This industry now occupies a position of major importance.
Elemental Phosphorus
Properties and Uses
White phosphorus is a highly poisonous white or lemon-colored waxlike solid having a melting point of 44.1° C., a boiling point of 280° C., and a specific gravity of 1.82. It is nearly insoluble in water, is slightly soluble in alcohol and certain other organic liquids, and dissolves readily in carbon disulfide and in ammonia. At 34° C. white phosphorus ignites spontaneously in air, evolving white fumes of phosphorus pentoxide (P2O5), which in turn combine with water to form orthophosphoric acid (H3PO4).
Red phosphorus is a dark-red amorphous solid obtained by heating white phosphorus to 250° C. out of contact with air. This variety has a specific gravity of 2.20 and a melting point of 590° C., is nonpoisonous, and does not ignite until heated to a temperature of approximately 260° C. in air. Red phosphorus is insoluble in water, carbon disulfide, and amnionia, but it is soluble in alkali solutions.
Elemental phosphorus has few industrial applications. During World War II, however, large tonnages were used in tracer bullets, incendiary bombs, and the production of smokescreens.
Poisonous pastes for exterminating rodents and household insect pests contain phosphorus, but in recent years these have been largely supplanted by various organic compounds.
Substantial quantities of white phosphorus are added to molten metals, such as tin and copper, to produce special alloys.
The main industrial application of red phosphorus is in the match industry. The striking surface of safety-match packages consists of red phosphorus, powdered glass, and glue, which ignite the head of a match drawn across the surface.
Most elemental phosphorus is converted to phosphoric acid for use in manufacturing numerous industrial products.
Manufacture of Phosphorus
The early methods of manufacturing limited quantities of elemental phosphorus were crude, cumbersome, and costly. Large tonnages are now produced in huge electric furnaces from raw materials that are plentiful.
Phosphorus also has been successfully produced in blast furnaces similar to those used in pig-iron manufacture, but in recent years the availability of relatively low-cost hydroelectric energy in some Southern and Western States has resulted in the general adoption of the electric-furnace process for elemental-phosphorus manufacture. Figure 2 shows a modern electric-furnace plant for making elemental phosphorus.
No matter which type of furnace is used, the fundamental principles involved are essentially the same decomposition at high temperature of calcium phosphate in the presence of silica and carbon to produce elemental phosphorus, carbon monoxide, and a molten silicate slag. The overall reaction in its simplest form is represented by equation (1).
Ca3(PO4)2 + 3SiO2 + 5O = 3CaSiO3 + P2 + 5CO………………………………………..(1)

Raw Materials and Preparation of Furnace Charge
This raw materials that comprise a furnace charge are phosphate rock, silica, and carbon in the form of coke breeze. They are so proportioned that there is ample carbon for reduction and enough silica to combine with the lime in the phosphate rock to form a readily fusible slag.
When a high-grade phosphate rock is used, silica is added in the form of gravel, but in recent years lower grade siliceous phosphates have been that obviate the necessity of adding substantial quantities of silica bubbles.
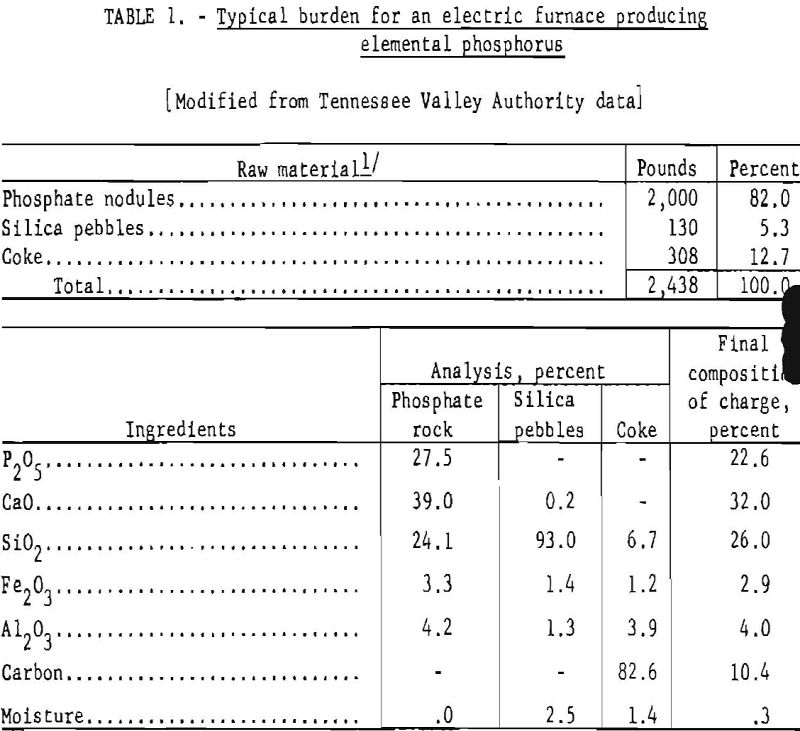
The furnace feed must be lumps or coarse granules to prevent bridging of the charge, insure steady and uniform descent of the materials, and maintain an open texture that permits ready evolution of phosphorus vapor and other volatile products without carrying over excessive quantities of dust or finely divided unreacted material into the auxiliary equipment. When finely divided phosphate rock is used, it is briquetted, sintered, or nodulized to the proper size. The methods of preparing various types of furnace charges and their relative advantages are discussed in a comprehensive engineering report by the Tennessee Valley Authority (TVA). Table 1 shows a typical burden for a phosphorus electric furnace using relatively high grade rock and the final composition of the charge.
Operating Steps
The carefully proportioned mixture of phosphate rock, silica, and coke breeze is fed continuously into the furnace and distributed in uniform layers around the carbon electrodes. As decomposition of the charge proceeds, a molten slag (relatively free from phosphorus) and a small quantity of ferro-phosphorus collect in the bottom of the furnace crucible and are drained off periodically through tap holes.
The volatile products, consisting of approximately 90 percent carbon monoxide, 7 percent phosphorus, and about 3 percent other gaseous products, led through a refractory-lined steel pipe into the Cottrell precipitators (maintained at a temperature well above the dewpoint of phosphorus), when the fine dust carried over by the furnace gases is collected.
From the hot precipitators the mixture of gases and phosphorus vapor enters the condensing system where it is sprayed with water and the bulk of the phosphorus condensed. The process water and elemental phosphorus drain into a sump, and the phosphorus forms a heavy liquid layer protected from oxidation by the overlying water. The process water, which is recirculated, is maintained at a temperature slightly above the melting point of phosphorus. A small part of the phosphorus is oxidized in the water, and soda ash is added intermittently to neutralize the dilute acid solution. The washed gases are then drawn through a vacuum pump, which removes the remaining phosphorus. From the vacuum pump the cleaned gas, consisting almost entirely of carbon monoxide, either passes to a storage tank or is utilized directly as a fuel. Figure 3 shows a flowsheet of an integrated plant for manufacturing both phosphorus and phosphoric acid.
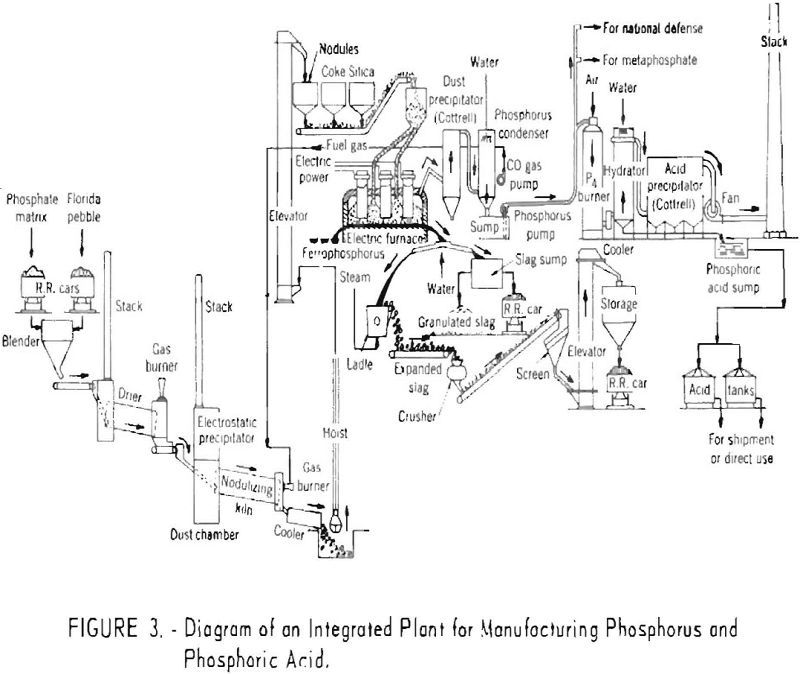
Phosphorus Recovery and Cost
Under average operating conditions, 90.5 percent of the phosphorus in the charge is volatilized, 6.5 percent is contained in the ferrophosphorus produced, and 3.0 percent remains in the slag. Of the phosphorus volatilized, 97 percent (nearly 88 percent of that in the furnace charge) is recovered.
The power consumption per ton of recovered phosphorus ranges from 12,000 to 13,000 kw.-hr., depending on the nature of the charge and the efficiency of operation. The estimated cost of producing elemental phosphorus in a plant comprising two 12,500-kw. furnaces producing 17,200 short tons of elemental phosphorus per year, as given by the TVA, is shown in table 2.
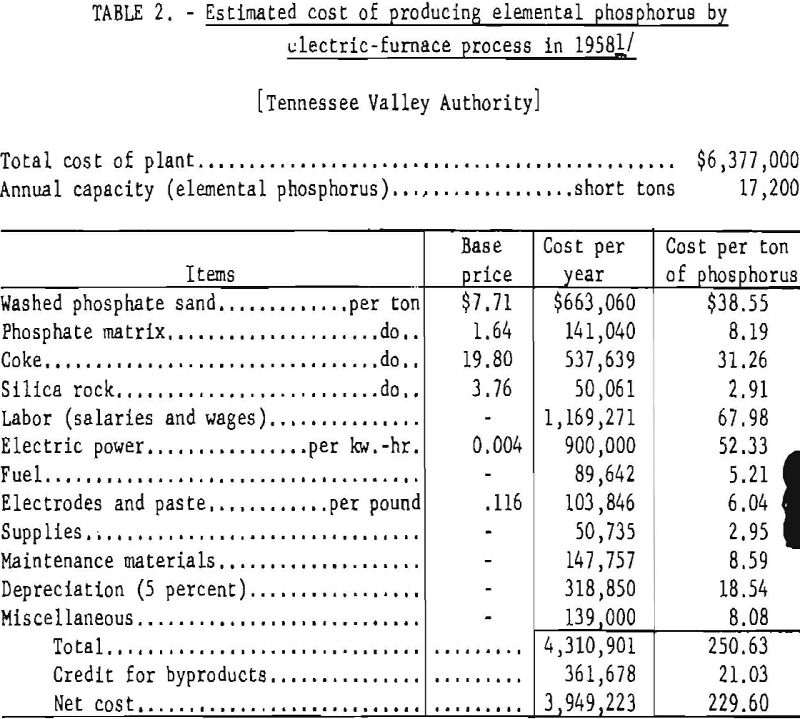
Byproducts
The byproducts from making phosphorus by the electric-furnace process are ferrophosphorus (an iron alloy containing about 23 percent of phosphorus), carbon monoxide gas, and a calcium silicate slag.
There is a limited market for ferrophosphorus in manufacturing special types of steel. The carbon monoxide gas is utilized as a supplementary fuel in preparing the phosphate charge for furnace treatment. The furnace slag may be crushed and sized for railroad ballast, ground finely and used as agricultural lining material, or converted into a lightweight concrete aggregate by treating the molten material with steam as it is tapped from the furnace. Although much of the fluorine in the furnace charge remains in the slag, a small percentage is volatilized and collected in the phosphorus condensing water as fluosllicic acid. To minimize corrosion of the condensing equipment, soda ash added to neutralize the acidity of the water. Vanadium is a potential byproduct that may be recovered in smelting western phosphate rock, but the vanadium content of the phosphate rock in Florida and Tennessee is too low to warrant recovery. Fluorine, uranium, chromium, and selenium also are possible byproducts of the elemental-phosphorus Industry.
Domestic Production
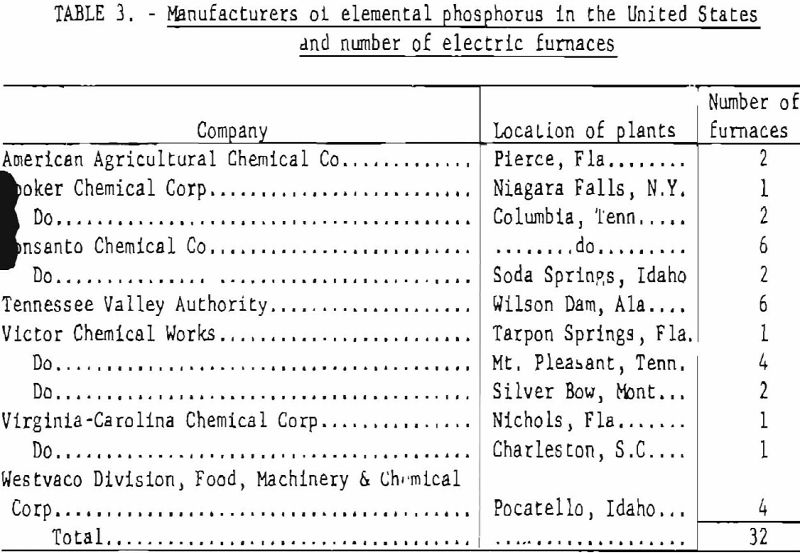
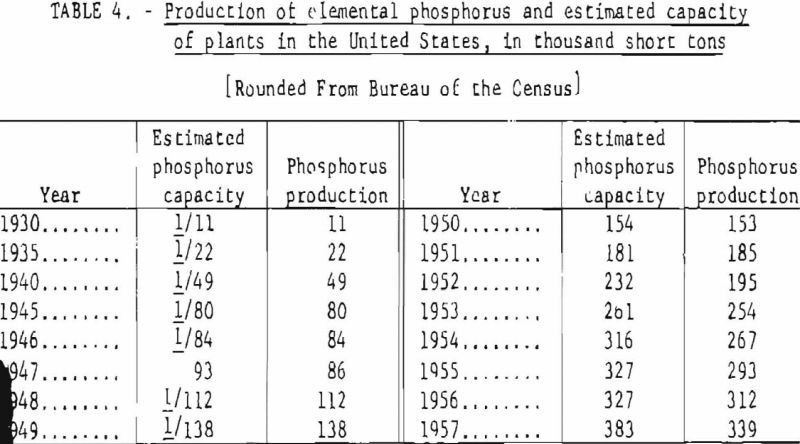
Table 3 gives the manufacturers of elemental phosphorus in the United States and the number and location of phosphorus plants. The total estimated capacity of these plants, as well as the actual annual production figures for 1930-57, are shown in table 4.
Virtually all manufacturers of phosphorus convert a large part of their output into phosphoric acid.
Phosphoric Acid
Orthophosphoric acid (H3PO4), usually referred to as phosphoric acid, is not only the most important acid of phosphorus but to some extent has displaced sulfuric acid in numerous Industrial applications. Although sulfuric acid is the cheapest and most widely used commercial acid, the sulfate radical (SO4) of the salts derived therefrom has little commercial value compared with that of the phosphate radical (PO4) of salts produced from phosphoric acid.
Phosphoric acid is produced by two methods:
- Oxidation of elemental phosphorus and hydration of the resultant P2O5.
- Decomposition of phosphate rock with sulfuric acid and filtration of the phosphoric acid from the insoluble residue.
Large tonnages of phosphoric acid are manufactured by both of these processes. Host of the acid derived from elemental phosphorus is used in manufacturing relatively pure phosphate products; the bulk of that produced by the sulfuric acid process is used in manufacturing phosphatic fertilizers.
Properties of Phosphate Rocks
Anhydrous phosphoric acid forms prismatic crystals, which readily deliquesce in the air. As ordinarily prepared, concentrated phosphoric acid is a clear, colorless, syrupy liquid (specific gravity, 1.83), which attacks most metals and bases, forming the corresponding phosphate salts. When heated to over 210° C., phosphoric acid loses part of its water of crystallization and is converted to pyrophosphoric acid (H4P2O7). On heating above 400° C more water is driven off, and the pyrophosphoric acid is converted to metaphosphoric acid (HPO3). The successive reactions are shown in equations (2) and (3).
2H3PO4 + heat = H4P2O7 + H2O……………………………………..(2)
H4P2O7 + heat = 2HPO3 + H2O……………………………………….(3)
Although phosphoric acid is used principally as an intermediate in manufacturing phosphate compounds, it has many direct uses. Substantial tonnages are added directly to irrigating water for fertilizer use and to ensilage and prepared stockfeeds to improve their nutrient qualities. Appreciable tonnages of chemical-grade phosphoric acid are used instead of citric and tartaric acids to impart tartness and palatability to soft drinks, jams, and jellies and as a defecating agent in refining sugar. Smaller quantities are used in the synthesis of certain dyes, in the manufacture of special types of glass, and as an ingredient of dental cement. In recent years phosphoric acid has proved an effective catalyst, promoting certain chemical reactions when absorbed on a porous medium such as diatomaceous earth. Substantial quantities of less pure acid are consumed in pickling metals and depositing rust-resisting coatings thereon.
Pyrophosphoric and metaphosphoric acids are little used in the free or uncombined state, but their salts (pyrophosphates and metaphosphates) have considerable commercial importance. The polymetaphosphates are used in fertilizers, detergents, lubricants, and drilling muds.
Manufacture of Phosphoric Acid From Elemental Phosphorus
The conversion of elemental phosphorus to phosphoric acid is effected in two stages: The phosphorus is first oxidized to P2O5, which is then reacted with water to form the acid, according to equations (4) and (5).
P4 + 5O2 = 2P2O5…………………………………………………………..(4)
P2O5 + 3H2O = 2H3PO4………………………………………………….(5)
Liquid phosphorus is pumped through a steam-jacketed pipeline to a water-cooled burner set into the top of a cylindrical combustion chamber. There it is mixed with an excess of air and oxidized at temperatures above 1,800° F. The domelike steel top of the combustion chamber is lined with acid-resisting refractory material. The walls and bottom arc constructed of graphite blocks set in asphalt cement. The combustion chamber is supported above ground level, and the bottom is laid in a stainless-steel pan 5 inches deep to collect any acid that may seep through the graphite blocks. To prevent oxidation of the graphite, the outside walls of the combustion chamber art cooled by a curtain of water, which is deflected near the base of the chamber to prevent it from entering any cracks in the chamber bottom.
The P2O5 and gaseous products are fed through an opening near the bottom of the combustion chamber into a graphite-lined cooler, where their temperature is reduced to about 350° F. They then enter a steel cylindrical hydrator lined with acidproof brick, where they are sprayed with water and the bulk of the P2O5 is converted to phosphoric acid. The cooled gases, containing suspended particles of acid, then enter a Cottrell electrical precipitator where the remainder of the acid is collected. The spent gase are discharged to the atmosphere through a tall stack.
Phosphoric acid of any desired concentration can be obtained by this process, but. it is customary to produce acid containing 75 to 85 percent H3PO4.
Estimated Cost of Production
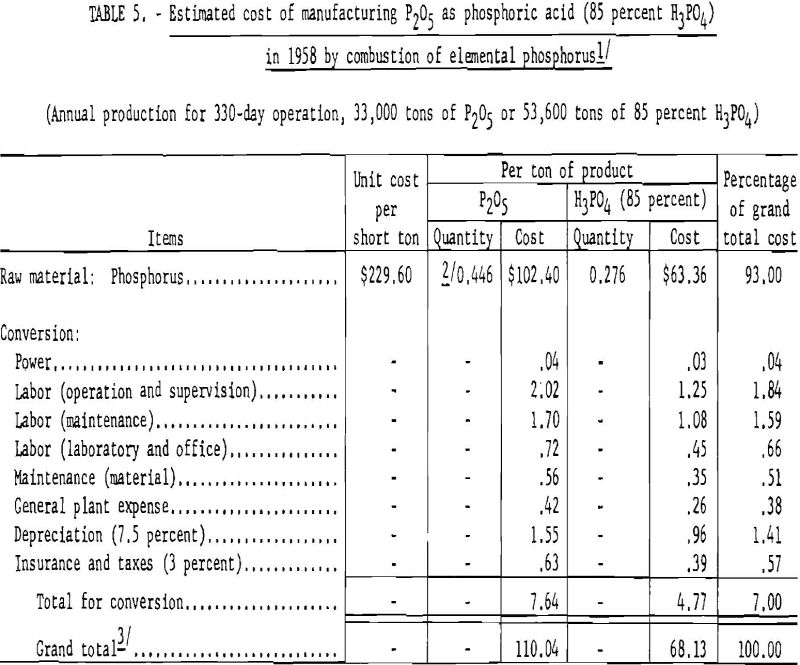
Table 5 gives the estimated cost of producing phosphoric acid from elemental phosphorus.
Manufacture of Phosphoric Acid by the Wet Process
Before extensive development of the electric-furnace process for making elemental phosphorus, the bulk of the phosphoric acid consumed for industrial, uses was manufactured by treating phosphate rock with sulfuric acid. This so-called “wet process” is still the main method utilized in producing phosphoric acid for fertilizer use, but it has been largely displaced by the electric-furnace process for high-grade chemicals and food products requiring relatively pure concentrated phosphoric acid.
The raw materials used in manufacturing phosphoric acid by the wet process consist of a medium-grade phosphate rock (usually 30 to 32 percent P2O5) and sulfuric acid. The main reaction is that between sulfuric acid and fluorapatite, producing phosphoric acid, calcium sulfate dihydrate (gypsum), and volatile fluorine compounds. The main reactions in their simplest form are shown in equation (6).
3Ca3(PO4)2·CaF2 + 10H2SO4 + 20H2O = 6H3PO4 + 2HF + 10(CaSO4·2H2O)…………..(6)
Actually the hydrofluoric acid is not evolved but reacts instantaneously with silica to form hydrofluosilicic acid, which is decomposed so that gaseous silicon tetrafluoride is liberated. As virtually all phosphate rock contains appreciable calcium carbonate and iron and aluminum compounds, more sulfuric acid must be added to decompose these impurities.
Modern plants for manufacturing phosphoric acid by the wet process are designed for continuous operation. A typical process is described as follows: Calculated quantities of concentrated sulfuric acid (60° to 66° B.) diluted with weak phosphoric acid and a recirculated slurry of partly decomposed phosphate rock are fed continuously to one or more agitator tanks, then discharged into the first of a series of mixing tanks to which weighed quantities of finely ground phosphate rock (90 percent minus-100-mesh) are continuously added. Mechanical stirrers mix these ingredients, and compressed air is introduced to cool the slurry. The gases evolved are treated with water to move the fluorine compounds. Complete decomposition of the rock and extraction of P2O5 is effected by a countercurrent system. The resultant phosphoric acid is separated from precipitated gypsum and other insoluble constituents by washing the slurry on vacuum filters. The phosphoric acid from the first filter (30 to 32 percent H3PO4) is termed “production acid.” This acid is concentrated by evaporation to 70 to 75 percent H3PO4 before it is used for making fertilizers. If the acid is to be used for high-quality phosphate products, certain purification steps are required. Under carefully controlled conditions the recovery of phosphoric acid in a well-operated plant ranges from 94 to 96 percent.
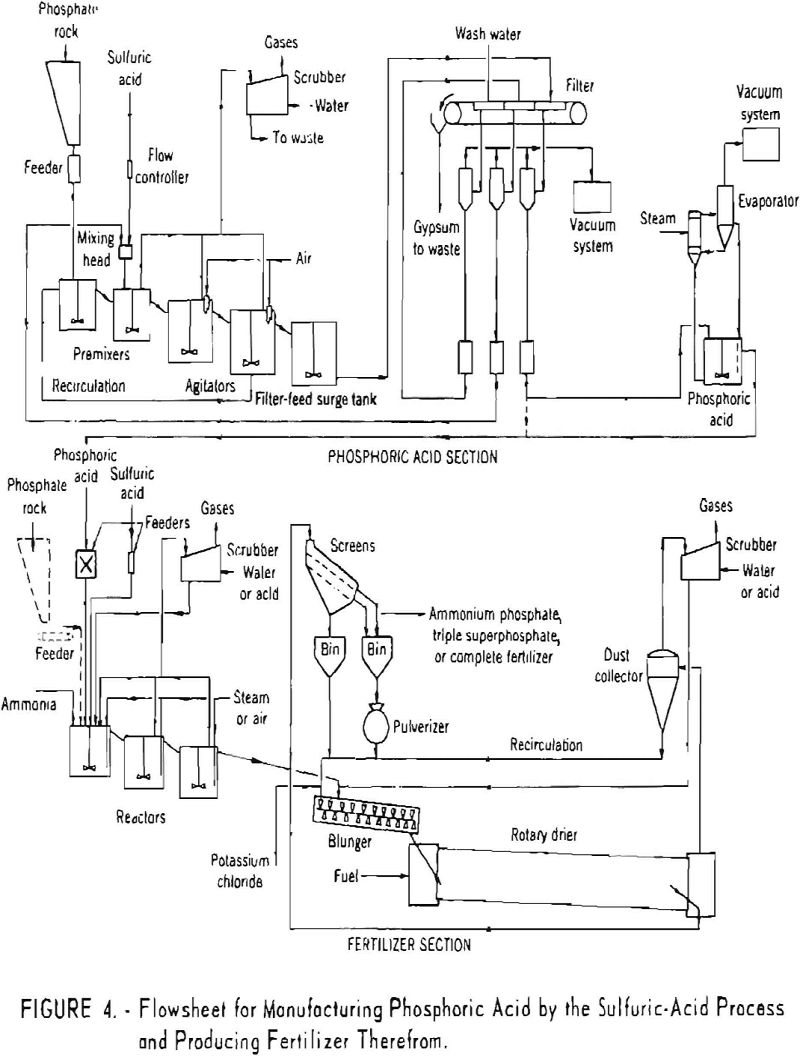
Figure 4 shows a flowsheet of the wet process for manufacturing phosphoric acid and the production of fertilizer therefrom.
Byproducts
The major byproducts that may be recovered in manufacturing phosphoric acid by the wet process are: Calcium sulfate, fluorine compounds, vanadium, and uranium. Chronium and selenium also have been reported in certain phosphate rocks.
Gypsum
Over 1 ton of gypsum is produced for each ton of phosphate rock consumed in making phosphoric acid. However, this gypsum is diluted with various impurities (chiefly silica and silicates) which detract from its value. It has been used to a limited extent in the manufacture of precast blocks for interior partitions of buildings, but no phosphoric-acid plants were utilizing this byproduct in 1957.
Fluorine Products
All commercial phosphate rock except that of recent origin contains 3 to 4 percent fluorine. When the rock is treated with sulfuric acid, a large part of this fluorine is evolved as silicon tetrafluoride (SiF4), which reacts with water to form fluosilicic acid and gelatinous silica. The fluosilicic acid may be filtered, concentrated, and marketed as such, or it may be converted into the fluosilicates of sodium, potassium, ammonium, or magnesium. Substantial quantities of sodium fluosilicate are converted into sodium fluoride. Fluosilicates have many industrial applications, including ceramics and insecticides. The advent of water fluoridation may greatly increase the demand for fluosilicic acid, as it is estimated that full fluoridation of domestic water supplies would require 23,000 tons of fluorine in the form of fluosilic, acid or its compounds.
Vanadium
Some phosphate rock contains a small quantity of vanadium oxide (V2O5). The phosphate deposits of the Western States, for instance, contain 0.07 to 0.40 percent vanadium as compounds soluble in sulfuric acid. A process for recovering vanadium from phosphate rock in marketable form has been successfully developed and used, but the operation was discontinued in 1955. Processing uranium ore yields more than enough vanadium to supply the present domestic demand.
Uranium
The Florida pebble-phosphate deposits and deposits in the Western States contain about 0.015 percent U3O8. When phosphoric acid is produced by the wet process, the uranium is dissolved and may be recovered by reduction of the acid solution and treatment with organic liquids that selectively extract the uranium compounds. The organic mixture is separated from the acid solution by centrifuging, and the uranium Is precipitated as a fluoride. The uranium precipitate then is purified and the organic solution recirculated.
In 1957 approximately 3.1 million tons of domestic phosphate rock was used to manufacture phosphoric acid by the wet process in the United States. If the average uranium oxide content is assumed to be 0.015 percent, the total U3O8 in this rock was 468 short tons.
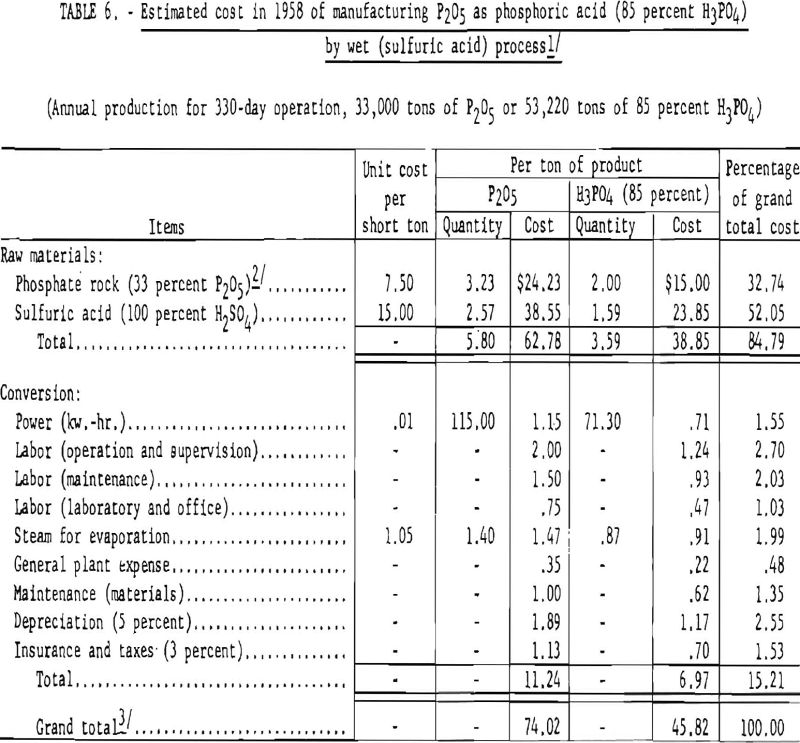
Estimated Cost of Production
Table 6 gives the quantities of phosphate rock and sulfuric acid required and the estimated cost of manufacturing phosphoric acid by the wet process, using Florida pebble-phosphate and sulfuric acid derived from elemental sulfur.
Domestic Production of Phosphoric Acid
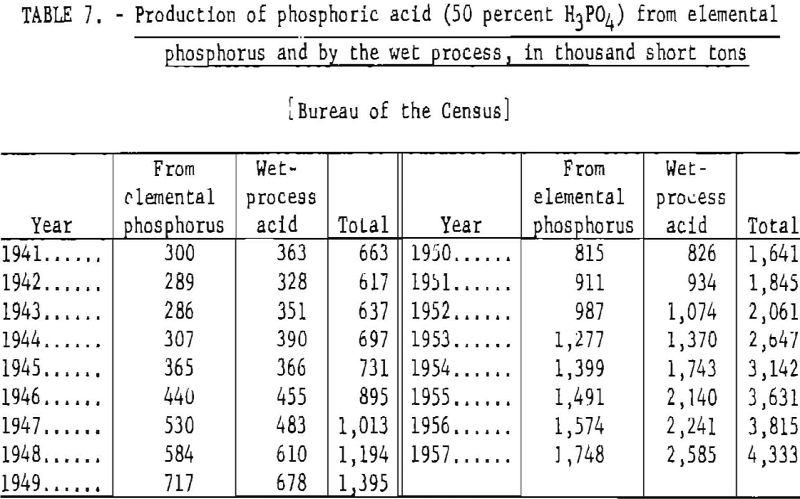
Table 7 gives the annual production of phosphoric acid (50 percent H3PO4) in the United States, 1941-57.
The manufacture of phosphoric acid from elemental phosphorus and by the wet process expanded from 696,570 short tons in 1944 to 4,332,090 short tons in 1957, an increase of more than 3.6 million short tons or over 400 percent. Except for 1947 and 1949, the quantity of acid produced by the wet process exceeded that derived from elemental phosphorus.
Phosphatic Fertilizers
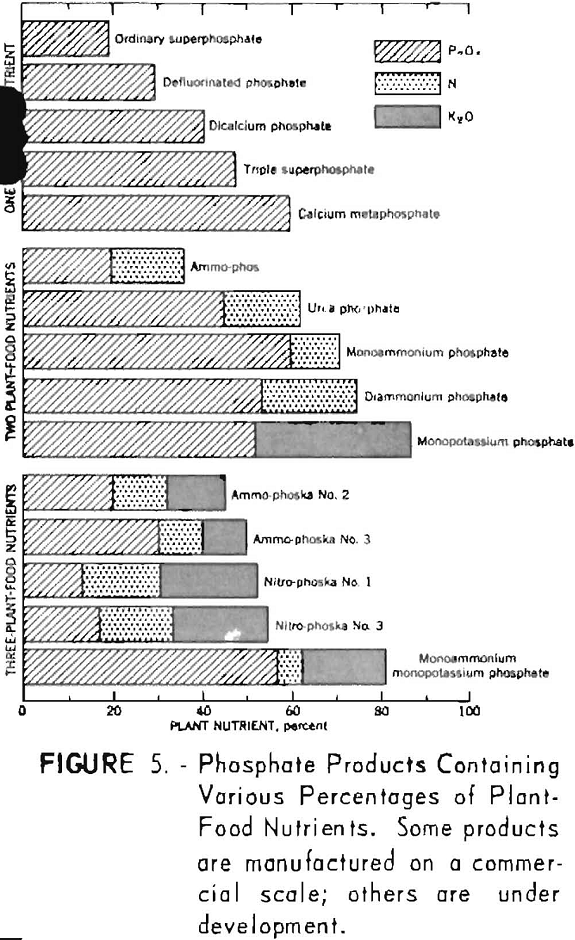
Although industrial uses for products derived from phosphate rock have expanded greatly during the past 25 years, the bulk of the phosphate rock produced was, and probably will continue to be, consumed largely for fertilizers. Phosphate fertilizers include a wide variety of products. Some are applied to the soil mainly for their phosphate value, whereas others consist of simple or double salts so mixed that they contain two or more of the main plant-nutrient elements (N, P, and K) in definite proportions. Within the past 25 years there has been an increasing trend toward the use of more concentrated fertilizer materials to offset increased manufacturing and transportation costs.
Figure 5 shows the composition of certain phosphate products containing one, two, or all three of the major plant-food nutrients.
Fertilizers derived from phosphate rock may be grouped conveniently in the following major categories:
- Water-soluble phosphates.
- Citrate-soluble or available phosphates.
- Citrate-insoluble phosphates.
The agricultural availability of phosphate products is considered as the sum of the contained water-soluble and citrate-soluble P2O5.
Water-Soluble Phosphates
Water-soluble phosphates comprise a number of highly soluble products. These products include ordinary (normal) superphosphate, concentrated superphosphate, ammonium phosphates, potassium phosphates, double salts of ammonium and potassium phosphates, and mixtures containing soluble phosphates and nitrates.
Normal Superphosphate
Normal superphosphate is a relatively dry, porous product consisting essentially of a mixture of monocalcium phosphate (a water-soluble phosphate salt) and calcium sulfate. Its available P2O5 content ranges from 18 to 22 percent, depending on the grade of rock and the free -moisture content of the final product.
The process consists of mixing finely ground phosphate rock containing 32 percent or more P2O5 with sulfuric acid (55° B.) in the ratio of 100 parts of rock to 85 parts of acid. The resultant slurry is stirred vigorously for several minutes and then discharged into a chamber or “den,” where the chemical reactions continue and the product hardens into a porous cake. After a period of 1 to 24 hours the cake is removed (usually by mechanical means) and the product transferred to a storage pile, where the chemical reactions are completed (curing) within several weeks. The product is then disintegrated, screened, and shipped (in bulk or bags) or mixed with potash and nitrogenous compounds to make complete fertilizers.
As nearly all phosphate rock contains different proportions of acid-consuming impurities, the chemical reactions involved are complex. The main reaction in making normal superphosphate is shown in equation (7).
Ca3(PO4)2 + 2H2SO4 + H2O = CaH4(PO4)2·H2O + 2CaSO4……………………………….(7)
Hydrofluoric acid, formed from the fluorine in phosphate rock, reacts with the contained silica to give silicon tetrafluoride (SiF4), which is evolved as a gas.
Host superphosphate plants are equipped with spray towers to absorb the silicon tetrafluoride, which reacts with water to produce fluosilicic acid and gelatinous silica. Many plants utilize the resulting solution to manufacture salts of fluosilicic acid.
Ordinary superphosphate requires no artificial drying and is the simplest phosphate fertilizer to manufacture. Where low-cost sulfuric acid is available and hauls to the market are short, superphosphate is usually the cheapest phosphate fertilizer to use. However, it is often more economical to ship concentrated superphosphate, if the cost of transportation to consuming centers is a major factor.
Concentrated Superphosphate
Concentrated superphosphate, as its name implies, is a product containing a higher percentage of P2O5 than ordinary or normal superphosphate. As the production of concentrated superphosphate entails the use of phosphoric acid, the plant is usually built adjacent to one that manufactures phosphoric acid by the wet process.
Concentrated superphosphate ranges in grade from 25 to 48 percent available P2O5. The lower grade is usually manufactured by decomposing phosphate rock with a mixture of sulfuric and phosphoric acids; the higher grade (also called triple superphosphate) is produced by treating the rock with phosphoric acid alone. Triple superphosphate contains little or no calcium sulfate.
The main reaction involved is shown in equation (8).
Ca3(PO4)2 + 4H3PO4 + 3H2O = 3CaH4(PO4)2·H2O……………………………(8)
No manufacture concentrated superphosphate using wet-process phosphoric acid, finely ground phosphate rock (more than 31 percent P2O5) is intimately mixed with phosphoric acid. The resultant slurry is discharged directly from the mixer onto a conveyor or directly into a reaction bin where it hardens to a relatively stiff mass. The partly cured product is transferred to a pile where the chemical reactions are completed (curing). Then the material is ground, or dried and ground, screened, and either shipped directly or mixed with potash and nitrogen compounds to make complete fertilizers.
To make granulated concentrated superphosphate, the slurry of phosphoric acid and phosphate rock is mixed with returned fines and crushed oversized material and put through a mixer and drier. The granules are then sized, the oversize and undersize material returning to the circuit.
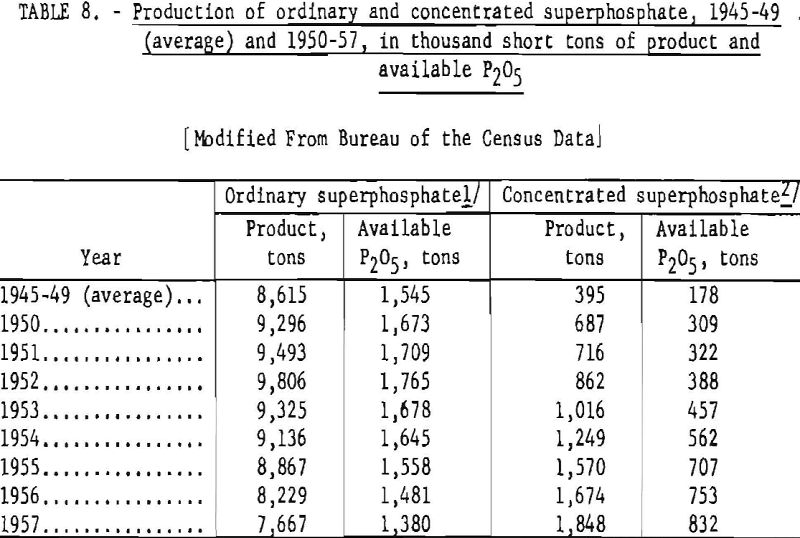
The production of ordinary superphosphate and concentrated superphosphate in the United States, 1945-49 (average) and 1950-57, is shown in table 8.
Ammoniated Superphosphate
Both ordinary superphosphate and concentrated superphosphate contain some free acid, as well as an acid salt (monocalcium phosphate) that can combine with substantial quantities of ammonia without decreasing the availability of the contained P2O5.
Ammoniated superphosphate is produced by mixing calculated quantities of either ammonia gas or a nitrogenous solution with the superphosphate in a rotating vessel. The free acid and part of the acid salt CaH4(PO4)2 react with the ammonia to produce dicalcium phosphate (CaHPO4) and one or more ammonium salts. The reactions in ordinary superphosphate containing sulfates are shown in equation (9).
CaH4(PO4)2·H2O + CaSO4 + 2NH3 = 2CaHPO4 + (NH4)2SO4 + H2O…………………………..(9)
When concentrated superphosphate containing little or no calcium sulfate is ammoniated, the reaction is as shown in equation (10).
CaH4(PO4)2H2O + NH3 = CaHPO4 + (NH4)H2PO4 + H2O……………………………………..(10)
Little ammoniated superphosphate is manufactured and sold. It is produced chiefly as one Ingredient resulting from the ammoniation of mixed fertilizers containing superphosphates.
Phosphate-Nitrate Fertilizers
Other phosphatic fertilizers containing water-soluble P2O5 include those obtained by treating phosphate rock with nitric acid or with mixtures of nitric and other mineral acids. The substitution of nitric for sulfuric acid as a reagent offers the advantage of producing mixtures containing two main plant nutrient elements (N and P). On the other hand, all proposed processes yield calcium or ammonium nitrate, both of which are hygroscopic and impart undesirable physical properties to the fertilizer. To obtain final products containing a minimum of the highly hygroscopic compound Ca(NO3)2, the nitrated materials are treated with controlled quantities of ammonia, resulting in the formation of ammonium nitrate (NH4NO3) and dicalcium phosphate (CaHPO4).
The main reactions of calcium phosphate and nitric acid, calcium phosphates and mixtures of nitric and sulfuric acids, and calcium phosphate and mixtures of nitric and phosphoric acids are shown in equations (11), (12), and (13).
Ca3(PO4)2 + 4HNO3 = CaH4(PO4)2 + 2Ca(NO3)2…………………………………………..(11)
Ca3(PO4)2 + 2HNO3 + H2SO4 = CaH4(PO4)2 + CaSO4 + Ca(NO3)2………………..(12)
Ca3(PO4)2 + 2HNO3 + 2H3PO4 = 2CaH4(PO4)2 + Ca(NO3)2…………………………..(13)
Liquid Fertilizers
Liquid fertilizers consist of solutions of one or more highly soluble fertilizer materials.
In recent years the consumption of liquid fertilizers in the United States has expanded at a phenomenal rate. In 1947, 47,136 short tons was used, and by 1957 the consumption rose to more than 1,300,000 tons (mainly nitrogenous fertilizers).
From the manufacturer’s point of view, liquid fertilizer mixtures containing phosphorus, nitrogen, and potassium have the following advantages over solid fertilizer mixtures of the same grade:
- The manufacturing equipment required is less costly.
- Less labor is involved in handling the materials.
- The problems of physical condition and segregation are largely eliminated.
From the farmer’s point of view, liquid fertilizers offer the advantage of being readily handled and uniformly distributed, but the final choice between liquid and solid phosphatic fertilizers depends chiefly on the cost of equivalent quantities of the plant-food constituents, Usually the per-unit cost of plant food in liquid form is greater than that in dry form.
Citrate-Soluble or Available Phosphates
The term “available phosphates” is applied to certain primary and secondary products resulting from the thermal and chemical treatment of phosphate rock. These phosphates, although virtually insoluble in water, bring about distinct increases in crop yields. Generally their agricultural availability is determined in the laboratory by their solubility in a neutral solution of ammonium citrate.
Because of their limited solubility, these products usually are more effective on acid soils high in organic matter. Under favorable conditions they dissolve at a rate commensurate with that at which crops extract the P2O5 from the soil solution. Some authorities contend that these less-soluble phosphates do not revert in the soil to an insoluble state as rapidly as the water-soluble phosphates, hence their effectiveness is spread over a longer period of time.
The fertilizer materials in this category comprise such products as calcium and potassium metaphosphate; calcined, fused, and defluorinated phosphate rock; and dicalcium phosphate.
Metaphosphates
Calcium metaphosphate (Ca(PO3)2) is a relatively new, highly concentrated fertilizer product containing 58 to 60 percent P2O5. It is manufactured and marketed by the TVA at Wilson Dam, Ala., by the reaction of P2O5 vapor and phosphate rock at high temperatures.
The process consists of spraying finely ground phosphate rock and elemental phosphorus into one end of a horizontal brick-lined cylindrical chamber with an excess of air to oxidize the phosphorus. The other end of the combustion chamber is connected with a vertical brick-lined tower filled with phosphate-rock briquets to absorb any surplus P2O5 that does not react with the rock dust in the combustion chamber.
At the high temperature (over 1,000° C.) developed by burning phosphorus the finely divided phosphate rock and phosphorus pentoxide react to form a molten slag consisting essentially of calcium metaphosphate, as shown in equation (14).
P4 + 5O2 + Ca3(PO4)2 = 3Ca(PO3)2…………………………………………………………(14)
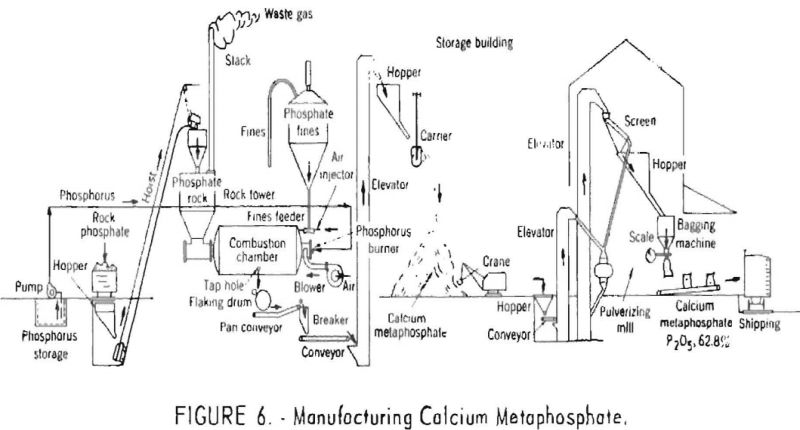
The molten calcium metaphosphate produced from the finely ground rock, as well as that formed in the absorption tower, collects in the bottom of the combustion chamber and flows continuously through a suitable taphole onto water-cooled steel rolls which chill and discharge it in the form of flakes. These flakes are subsequently ground and packed in bags for shipment. A flowsheet of the latest type of plant used in manufacturing calcium metaphosphate is shown in figure 6.
Several methods have been proposed for manufacturing potassium metaphosphate, but it has not yet been produced on a commercial scale.
One method is to react phosphorus pentoxide with potassium chloride in the presence of water or phosphoric acid; however, the process is intricate and difficult to control. The reaction is represented by equation (15).
P4 + 5O2 + 4KCl + 2H2O = 4KPO3 + 4HCl……………………………………….(15)
Potassium metaphosphate is the only compound known containing 100 percent primary plant nutrients (60 percent P2O5 and 40 percent K2O).
Dicalcium Phosphate
Dicalcium phosphate can be manufactured directly by partly neutralizing phosphoric acid or monocalcium phosphate with lime or limestone, but little or none is produced by this process for fertilizer use because of the cost. However, when phosphate rock is decomposed with nitric or hydrochloric acid, the P2O5 present in the products usually is converted into dicalcium phosphate by treatment with ammonia. Dicalcium phosphate may be left in the resultant mixture or separated by filtration. Moreover, in ammoniating superphosphate, appreciable quantities of dicalcium phosphate are formed and remain in the product.
Because of its greater value as an animal-food supplement, dicalcium phosphate that is virtually free from fluorine is being manufactured in substantial quantities for use as an additive to stockfced, particularly in areas where ground and steamed bone is in short supply.
Defluorinated and Fused Phosphate Rock
Defluorinated phosphates are obtained by calcining or fusing phosphate rock with silica and/or phosphoric acid in the presence of water vapor. At temperatures ranging from 1,400° to 1,600° C., the bulk of the fluorine is evolved, and the P2O5 is rendered citrate soluble. Whereas defluorinated phosphate rock has proved to be an efficient fertilizer on acid soils, its effectiveness on alkaline soils is somewhat doubtful. Like dicalcium phosphate, defluorinated phosphate rock brings a high price as an ingredient of stockfeed; hence relatively little is used as fertilizer in the United States.
Similar products are obtained by calcining or smelting phosphate rock with sodium and magnesium salts, which break up the apatite molecule and either drive off the fluorine or combine with it so that the final product contains P2O5 in a citrate-soluble or agriculturally available form. Substantial quantities of these products are manufactured for fertilizer in some European countries, but only limited quantities have been produced in the United States.
Citrate-Insoluble Phosphates
Substantial tonnages of finely ground phosphate rock are applied directly to the soil. They are insoluble in water and only slightly soluble in carbonated water or a neutral solution of ammonium citrate. Nevertheless, when uniformly distributed in acid soils they expose such a large surface to the action of the soil water that the contained P2O5 is made available to crops over a long period of time.
Finely ground phosphate rock is a soil builder for supplying P2O5 soils low in phosphate minerals. Table 9 shows annual consumption of finely ground phosphate rock for direct application to soils, 1945-57.
Other Industrial Products Derived From Phosphate Rock
In addition to the immense tonnage of phosphate rock consumed by the fertilizer industry, large quantities are used to manufacture phosphate chemicals to meet an expanding demand.
The fact that most of these products are relatively high grade chemicals that can best be derived from white phosphorus accounts for the great increase in the number and size of electric-furnace plants for extracting elemental phosphorus.
Inorganic Phosphates
As orthophosphoric acid contains three replaceable hydrogen atoms, it may be used to produce a series of primary, secondary, and tertiary salts as typified by the following reactions:
Primary salt – H3PO4 + NaOH = NaH2PO4 + H2O
Secondary salt – H3PO4 + 2NaOH = Na2HPO4 + 2H2O
Tertiary salt – H3PO4 + 3NaOH = Na3PO4 + 3H2O
Although many inorganic compounds may be produced by treating various bases with phosphoric acid, the phosphate salts having the widest industrial applications are those of calcium, ammonium, and sodium.
Calcium Phosphates
Substantial tonnages of relatively pure monocalcium phosphate (CaH4(PO4)2·H2O), dicalcium phosphate (CaHPO4.2H2O), and tricalclum phosphate (Ca3(PO4)2) are consumed for uses other than fertilizer.
Calcium phosphates are produced by the direct treatment of lime with phosphoric acid; the successive reactions are given in equations (16), (17), and (18).
2H3PO4 + CaO = CaH4(PO4)2.H2O……………………………(16)
CaH4(PO4)2.H2O + CaO = 2CaHP04.2H2O………………..(17)
2CaHPO4.2H2O + CaO = Ca3(PO4)2 + 3H2O……………..(18)
In manufacturing monocalcium phosphate, weighed quantities of crushed lime and phosphoric acid (75 percent H3PO4) derived from elemental phosphorus are intimately mixed in a ceramic-lined container. The batch is maintained at a temperature of about 100° C., and much of the free moisture is evolved during the reaction. The crystalline product is dried under controlled conditions, crushed, and screened. The coarser granular particles are mixed with sodium bicarbonate and starch to produce baking powder, and the finer material, is used as a leavening agent in self-rising flours.
In recent years, anhydrous monocalcium phosphate containing small quantities of certain metallic ions has been developed. When such a product is carefully heated, metallic ions form a coating over the granules of monocalcium phosphate, rendering them less absorbent of atmospheric moisture and better adapted for certain types of baked products in which a delayed leavening action is desirable. Small quantities of monocalcium phosphate are also used in medicinal preparations and as an ingredient of tonics designed to supply both lime and phosphoric acid.
As shown in the section on phosphatic fertilizers, dicalcium phosphate (CaHPO4.2H2O) is formed when superphosphates are treated with ammoniacal solutions. This compound also is precipitated when ammonia is added to the mixtures resulting from decomposition of phosphate rock with nitric acid; however, the dicalcium phosphate thus produced usually remains in the product as a fertilizer constituent.
Dicalcium phosphate for other industrial purposes is manufactured directly by the same general process as that used in producing monocalcium phosphate, except that a larger proportion of lime or limestone is used. Substantial quantities of dicalcium phosphate are also obtained as a byproduct in manufacturing gelatin and glue from bones.
The total production of dicalcium phosphate in 1956 was about 147,000 short tons, of which over 80 percent was consumed for stock feeding. Other uses included pharmaceuticals, mild abrasives, leavening agents, and an ingredient in special types of glass.
Tricalcium phosphate is manufactured by mixing phosphoric acid with an emulsion of hydrated lime (Ca(OH)2) in such proportions as to form a slightly basic slurry. The slurry is then pumped to a filter press and washed with water. The damp cake is dried and milled. Tricalcium phosphate is used principally as a mild abrasive in tooth pastes and as a conditioning agent to minimize the caking of table salt and powdered sugar. The production of tricalcium phosphate for industrial uses is relatively small compared with the production of other phosphates of lime. The annual production for all purposes is estimated at 5,000 short tons.
Ammonium Phosphates
Monoammonium phosphate (NH4H2PO4) and diammonium phosphate ((NH4)2HPO4) are crystalline salts produced directly by absorbing ammonia gas in phosphoric acid. The successive reactions are shown in equations (19) and (20).
H3PO4 + NH3 = NH4H2PO4………………………………………..(19)
NH4H2PO4 + NH3 = (NH4)2HPO4………………………………(20)
Diammonium phosphate is less stable than monoammonium phosphate and decomposes at temperatures above 100° C.; hence, certain precautions must be taken to prevent loss of ammonia by overammoniation or by drying the crystals at too high a temperature.
High-grade monoammonium phosphate is made by treating phosphoric acid (75 percent H3PO4) derived from elemental phosphorus with ammonia gas in a saturator similar to that used in manufacturing ammonium sulfate. A pasty mass results, from which the crystalline salt is obtained by cooling, centrifuging, and drying.
Diammonium phosphate is usually manufactured in two stages, but the TVA has developed a continuous, single-stage saturation process for producing diammonium phosphate crystals. The TVA process involves the continuous crystallization of diammonium phosphate from a saturated mixture of phosphoric acid derived from elemental phosphorus and gaseous ammonia under closely controlled temperature and acidity. The crystals of ammonium phosphate are centrifuged, washed, and dried, and the saturated solution is returned to the circuit.
Where wet-process phosphoric acid is used, impurities such as iron, aluminum, and fluorine make it difficult to obtain pure crystalline diammonium phosphate, but mixtures of monoammonium and diammonium phosphate that are stable at elevated temperatures and under humid conditions can be manufactured in granulated form.
The ammonium phosphates are being utilized to an increasing extent to manufacture concentrated fertilizers, but they have numerous other important industrial applications.
When highly heated, the ammonium phosphates evolve ammonia and are converted into tacky masses; this fact, coupled with their high solubility, renders them useful in fire-retardant compositions for impregnating fabrics and other combustible materials.
Monoammonium phosphate is used in yeast-culture media and to a limited extent in the dye industry. Diammonium phosphate is used in ammoniated tooth pastes and mouthwashes.
Sodium Phosphates and Their Derivatives
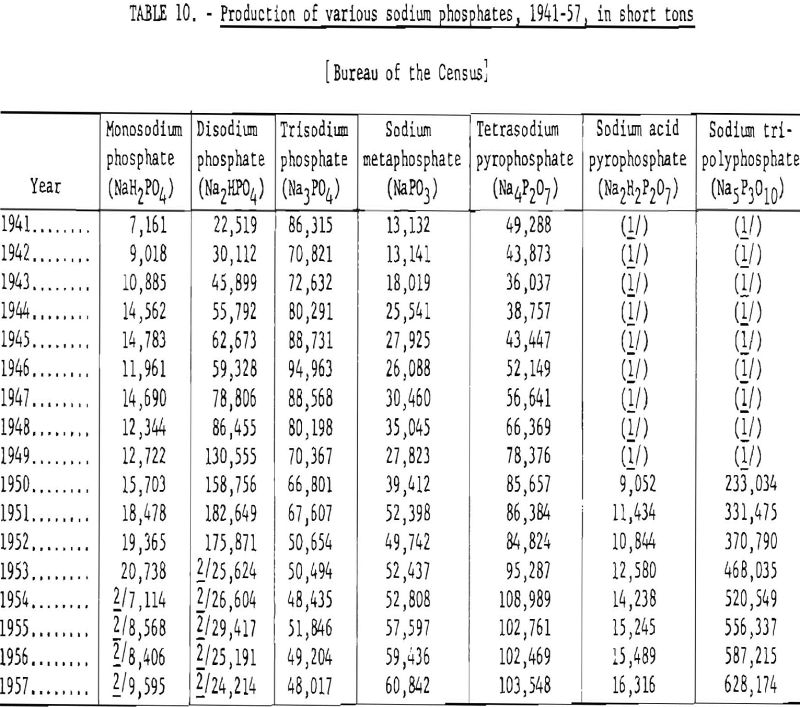
By far the most important inorganic phosphate compounds used for purposes other than fertilizers are the sodium phosphates. Monosodium phosphate (NaH2PO4), disodium phosphate (Na2HPO4), and trisodium phosphate (Na3PO4) are produced in large quantities by the direct treatment of soda ash or sodium hydroxide with phosphoric acid. The successive reactions are shown in equations (21), (22), and (23).
2H3PO4 + Na2CO3 = 2NaH2PO4 + CO2 + H2O……………………………(21)
2NaH2PO4 + Na2CO3 = 2Na2HPO4 + CO2 + H2O……………………….(22)
Na2HPO4 + NaOH = Na3PO4 + H2O…………………………………………..(23)
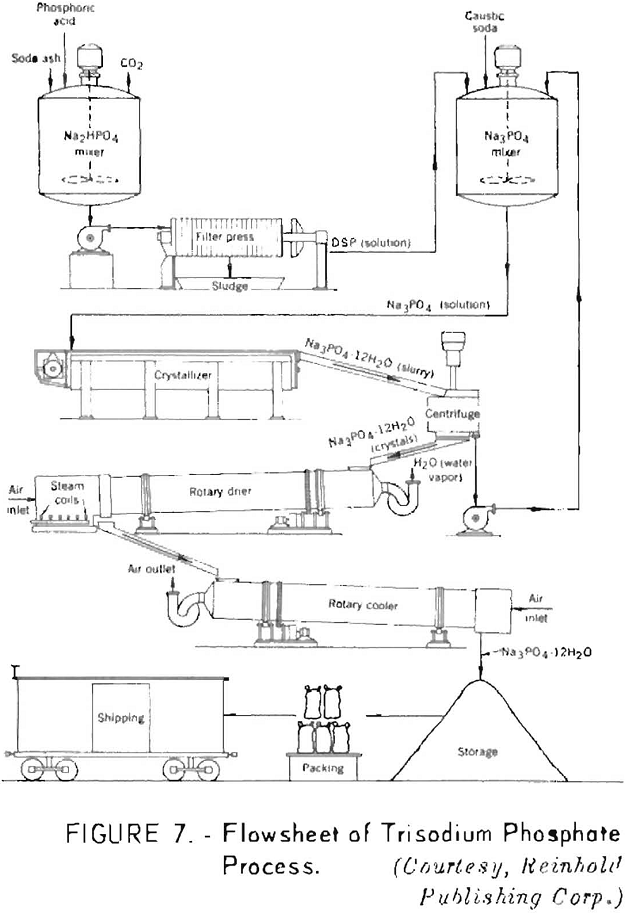
In manufacturing trisodium phosphate, the third hydrogen atom must be displaced by sodium hydroxide, as disodium phosphate does not react with soda ash. The solid salts for commercial use are obtained by direct crystallization and drum drying.
Figure 7 shows a flowsheet of the trisodium phosphate process.
The main direct uses for monosodium phosphate are in boiler-water treatment and in textile processing to control the pH. Disodium phosphate is also used for these purposes; in addition, substantial tonnages are used in tanning leather, weighting silk, and processing cheese and in certain medicinal products.
Virtually all of the trisodium phosphate marketed as a detergent contains 12 molecules of water of crystallization.
The bulk of the monosodium and disodium phosphates are heat-treated and converted into pyrophosphates, metaphosphates, or polyphosphates. The last-named category includes a large number of complex products that have assumed great industrial importance.
Sodium acid pyrophosphate (NaH2P2O7), which is derived from heating monosodium phosphate under carefully controlled conditions, is used as a leavening agent. It has mild acid properties and, when mixed with sodium bicarbonate, is well adapted for prepared cake and doughnut mixes. A small quantity of sodium acid pyrophosphate is used in electroplating.
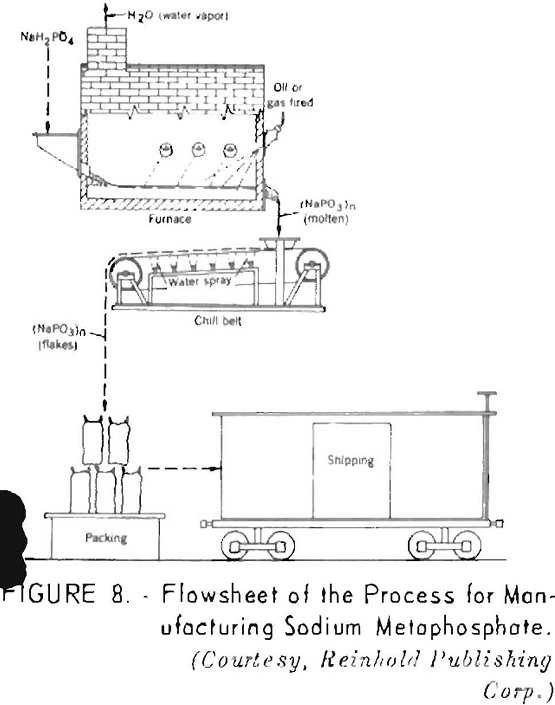
When heated to a higher temperature, monosodium phosphate is converted to sodium metaphosphate (NaPO3), a glassy product that is widely used in treating hard waters and as a soap builder to enhance the washing properties of soaps. A flowsheet of the process for manufacturing sodium metaphosphate is shown in figure 8.
Tetrasodium pyrophosphate (Na4P2O7) is produced by calcining disodium phosphate at a temperature above 300° C. to drive off its water of constitution. Tetrasodium pyrophosphate is mixed with soap powders and detergents to increase their effectiveness.
Other sodium polyphosphates are manufactured by calcining mixtures composed of different proportions of monosudium and disodium phosphate. Before 1950, the production statistics for sodium polyphosphates were included with those of sodium and disodium phosphate.
Sodium polyphosphates are used extensively to treat boiler feed water, and for purposes where a detergent combining mildness and effectiveness is required.
Table 10 gives the annual production of sodium phosphates, 1941-57.
Phosphorus Chlorides, Sulfides, and Pentoxide
Although a wide variety of inorganic phosphorus compounds other than phosphoric acid and its salts can be produced from elemental phosphorus, the more important of these industrially are the phosphorus chlorides, the phosphorus sulfides, and phosphorus pentoxide.
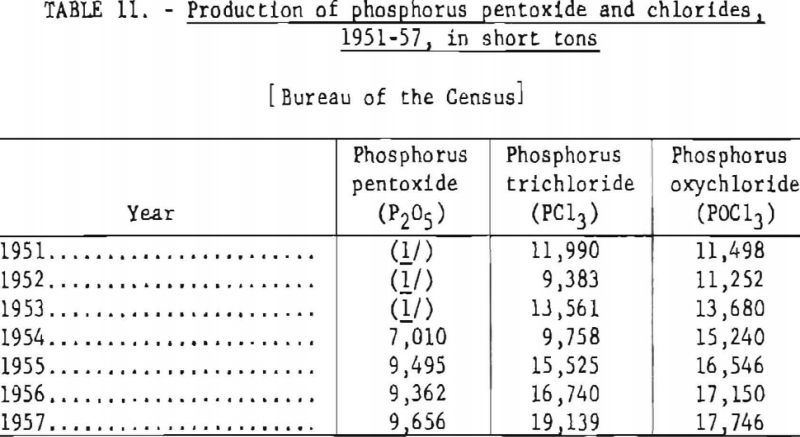
There are three commercially important chlorides of phosphorus: Phosphorus trichloride (PCl3), phosphorus pentachloride (PCl5), and phosphorus oxychloride (PClO3). Phosphorus tri-chloride and oxychloride are liquids at ordinary temperatures; phosphorus pentachloride is a crystalline solid.
The chlorides of phosphorus are manufactured by the direct union of the elements (P and Cl) care being taken to control the heat of the reaction by refluxing part of the product. The products are subsequently purified the trichloride by distillation and the pentachloride by recrystallization. Phosphorus oxychloride is produced by bubbling oxygen through phosphorus trichloride or by mixing phosphorus pentachloride with phosphorus pentoxide.
The chlorides of phosphorus are used in the manufacture of organic phosphorus compounds. Phosphorus trichloride reacts with alcohols or phenols to form phosphite esters, and phosphorus oxychloride under similar conditions yields phosphate esters.
Both phosphorus trichloride and phosphorus pentachloride are used as catalysts in chlorinating certain organic compounds, particularly acids, to produce acid chlorides. Phosphorus pentachloride in a more active chlorinating agent than the trichloride.
The two commercially important sulfides of phosphorus are phosphorus tri-sulfide (P4S3) and phosphorus pentasulfide (P4S10), both of which are yellow solids.
Like phosphorus chlorides, phosphorus sulfides are made by direct union of the elements (P and S). Properly proportioned charges of the two materials are stirred continuously in closed iron containers maintained at temperatures managing from 320° to 380° C. until the reaction is complete. The product is given ground to a powder and purified by washing it with the proper solvent to remove any excess sulfur or phosphorus.
Phosphorus trisulfide is the essential ingredient in the heads of the kitchen “strike anywhere” matches and has entirely replaced elemental phosphorus, previously used for this purpose. Small quantities of this compound are also used in making fireworks.
Phosphorus pentasulfide is used to prepare sulfur-bearing organic compounds and as an ingredient of certain flotation agents. Recently, it has proved an effective additive to lubricants. According to Van Wazer considerable work is being done on developing the organic chemistry of its reaction products.
Three crystalline varieties of phosphorus pentoxide (P2O5) have been identified. All three forms are decomposed by water and converted successfully into metaphosphoric, pyrophosphoric, or orthophosphoric acid as shown in equations (24), (25), and (26).
P2O5 + H2O = 2HPO3…………………………………………(24)
2HPO3 + H2O = H4P2O7…………………………………….(25)
H4P2O7 + H2O = 2H3PO4………………………………….(26)
Phosphorus pentoxide is prepared commercially by combustion of elemental phosphorus in an excess of dried air to insure complete oxidation. The product is collected as a white, finely divided, crystalline solid in large externally cooled chambers. The product is packed in hermetically sealed containers to prevent it from absorbing moisture.
Because of its great affinity for water, phosphorus pentoxide is one of the most efficient drying agents known, and this is its main industrial application. It is also used in preparing certain organic phosphates, as it reacts with alcohols to form phosphoric acid esters.
Table 11 shows the annual production of phosphorus chlorides and phosphorus pentoxide in the United States, 1951-57. It is estimated that 3,500 short tons of elemental phosphorus is consumed annually in the manufacture of phosphorus sulfides.
Organic Compounds of Phosphorus
Compared to inorganic phosphates, organic compounds of phosphorus are manufactured in relatively small quantities, but in recent years their field of usefulness has broadened considerably.
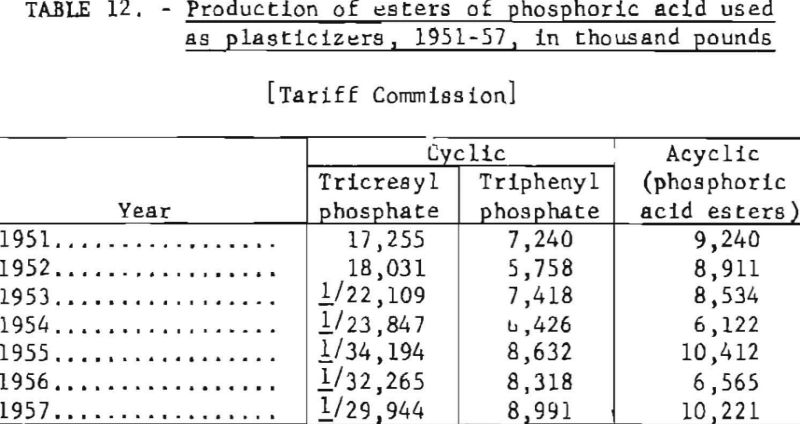
The more important organic phosphates produced on a commercial scale are the esters of phosphoric acid. These esters are prepared by mixing phosphorus pentoxide (P2O5) or phosphorus oxychloride (POCl3) with the appropriate alcohol or phenol in a closed vessel under carefully controlled conditions.
Certain alkyl and aryl phosphates made in this manner are excellent plasticizers. A plasticizer is a liquid or low-melting-point solid which when added to a resin modifies one or more of the physical characteristics of the resin. The most important function of a plasticizer is to increase the plasticity of a resin so that it may be molded into any desired form. The mixture should be resistant to aging, weathering, and fire.
The two principal organic phosphorus compounds used as plasticizers are tricresyl and triphenyl phosphates. Their use has expanded substantially with the phenomenal growth of the plastic industry. These organic phosphates are being used in increasing quantities as additives in hydraulic fluids and other oils. They not only serve as dispersing agents, corrosion inhibitors, and antioxidants but also are lubricants.
In recent years some esters of phosphoric acid have proved to be highly efficient insecticides in controlling aphids and mites. One such compound is tetraethyl pyrophosphate (TEPP), the production of which reached 515,000 pounds in 1955.
The organic reaction products of phosphorus pentasulfide, such as the dialkyl ester of dithiophosphoric acid (R3PO2S2) and its related salts, are effective flotation agents for recovering certain metals from their ores.
Table 12 gives the production of some of the more important phosphoric acid esters, 1951-57.
Phosphate Products
The phenomenal expansion of the phosphate industry during the past 15 years or more may be attributed to the following factors:
- The increased use of phosphatic fertilizers.
- New and expanded outlets for phosphate-products other than fertilizers.
- Improvement in techniques for processing phosphate rock.
According to figures of the U.S. Department of Agriculture, the harvested acreage of crops in the continental United States in 1955 was only about 2 percent greater than in 1940, yet the output of agricultural products in 1955 not only was sufficient to meet the needs of a population that had increased more than 25 percent but provided a large surplus.
Although the greater yield of crops per acre was due to a number of factors, it is highly significant that the annual consumption of fertilizers in this 15-year period rose from 8,656,000 to 22,500,000 tons, an increase of 160 percent.
New industrial uses for relatively pure inorganic phosphate salt, particularly in the detergent field, have provided a large market for such products. Certain organic phosphates also have been developed that are of great importance in the plastic industry, as additives to motor fuel, and in the manufacture of insecticides.
Improvements in mining and processing techniques have allowed the utilization of lower grade phosphatic raw materials that formerly were considered economic. More efficient procedures in the electric smelting of phosphate ores for recovering phosphorus and subsequently converting it into phosphoric acid are among the outstanding developments contributing to the wider use of phosphate compounds. Even when such plants are far from consuming centers, the shipment of elemental phosphorus and concentrated phosphoric acid to distant markets has proved economically feasible.
The outlook for further expansion of the phosphate industry is favorable although it is doubtful if the upward trend will continue at the rate of the past 15 years.
Crop production, however, must keep pace with the increasing population of the United States, and this can be accomplished on a limited acreage only by intensive fertilization.
Although there is little doubt that other industrial uses will be found for both organic and inorganic phosphates, it seems unlikely that such uses will create a demand comparable to that resulting from the use of phosphates in detergents.
There are, however, promising possibilities of effecting further improvements in processing techniques. Moreover, better recovery and utilization of byproducts would reduce the net cost of manufacturing phosphate compounds and provide an additional incentive to expand manufacturing facilities.
