Table of Contents
Many factors contributed to the decision to build a radiochemical laboratory at the Salt Lake City Metallurgy Research Center to investigate the application of radioactive isotopes and chemical compounds in extractive metallurgy. These reasons included the notable success achieved in biological sciences with radioisotopes, the development of reliable instruments and methods for identifying the isotopes and measuring their activities, and the commercial availability of radioisotopes in a variety of forms and known activities. The paramount reason, however, was the realization that better methods were needed for guiding and quantifying research to resolve the complex extractive metallurgy problems under study at the Research Center.
This is the first of a series of reports describing the application of radioisotopes and radioactivity techniques in extractive metallurgy. The report includes a discussion of licensing and safety requirements, a general description of the laboratory and the methods used, a definition of the nature and scope of the research program, and a synopsis of the more important problems studied. Subsequent reports will concern radioactive techniques for solving specific problems in mineral dressing, hydrometallurgy, and pyrometallurgy.
Licensing Requirements
In order to purchase radioactive isotopes for research purposes, a byproduct material license must be obtained from the Atomic Energy Commission (AEC), Division of Licensing and Regulation. Several conditions must be fulfilled. A laboratory isolated from the usual working areas in the building must be provided. Provision must be made for safe storage of radioisotopes and for easy cleaning of floor, walls, and working areas. In addition to describing the facilities and equipment, the application must cite the training and experience of the personnel assigned to work with radioisotopes and specify the kind and maximum quantities of isotopes that will be stored at any time in the laboratory. A safety program that will govern operations in the laboratory must be formulated; a radiation safety officer with specialized training appointed; and an accounting system devised for the radioisotopes bought, used, and disposed of in the work, Several publications relating to the design of radiochemical laboratories and the safe handling of radioactive materials are listed in the selected bibliography.
Hazards and Precautions
Much has been written about the hazards of exposure to radiation. For the purpose of this paper, it is sufficient to state that the trend is toward ever lower maximum radiation-exposure limits and stricter safety measures and controls. Regulations are set forth in the Federal Register. Copies are available from the Superintendent of Documents, Washington 25, D. C.
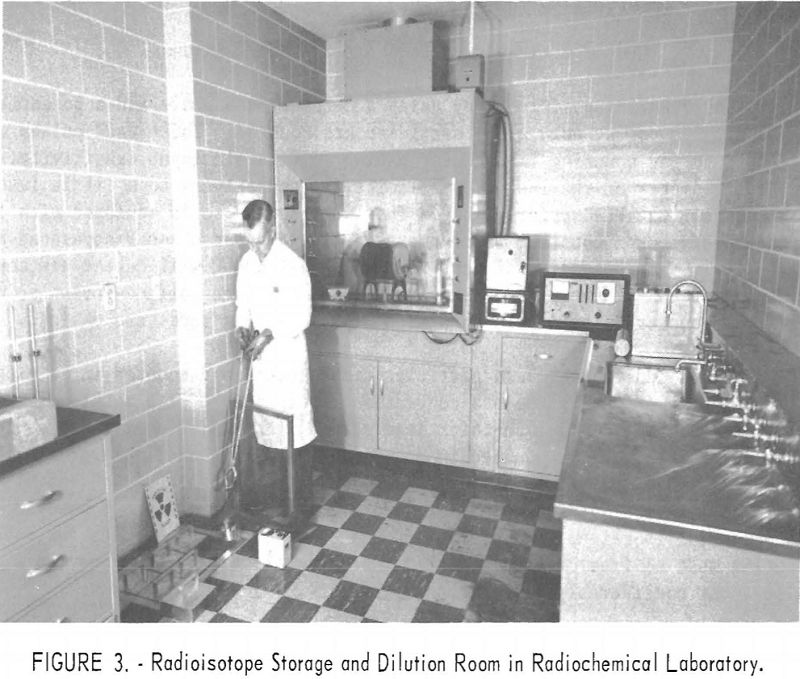
The laboratory at the Salt Lake City Metallurgy Research Center is designed for low-level radioactivity work. The present license limits available isotopes to 100 millicuries and individual radioisotopes to a few millicuries. The separate isotopes, as needed for research, are diluted in the isotope storage room to a few microcuries for the experimental work in the research laboratory.
Radiochemical Laboratory workers are given periodic physical examinations, must wear film badges or dosimeters that record the accumulated radiation exposure; and are required to observe safety rules governing laboratory operations; and to receive training in the use of radioisotopes and specialized equipment and instruments.
Radiation exposure has been very low. The only measurable amount has been received during the storage and dilution of the isotopes. The quantity of isotope needed for the individual experiments is too small, usually less than a microcurie of activity, to give any measurable exposure. The working distance between the operator and the experimental test, usually 3 feet, also reduces the exposure to a safe level, even though considerable time may be required to complete the experiment. Safety is enhanced by rubber gloves and by unusual care in maintaining clean equipment and preventing contamination.
Laboratory personnel formulate safe working procedures for all new experiments, and these methods are stated on an instruction sheet before testing is initiated. Hurrying or working under pressure is discouraged.
The greatest danger in radioisotope research results from the possible spillage of materials during dilution or some other handling step in the test procedure. Spillage in working areas would be serious, chiefly because decontamination may require extraordinary delays for washing walls, removing and relaying floor tile, or dismantling and cleaning equipment and instruments.
Availability of Radioisotopes
Several thousand radioactive elements and compounds of specific concentration and activity are now available for research purposes. Radioisotopes produced in a nuclear reactor can be purchased from the Oak Ridge National Laboratory, Isotopes Sales Department, P. O. Box X, Oak Ridge, Tenn. A catalog entitled, “Radioisotopes,” lists the prices, properties, and services available at Oak Ridge. Many so-called “labeled” or “tagged” inorganic and organic compounds synthesized from radioisotopes are manufactured and marketed by several commercial firms. The properties and prices of the compounds now available are listed in a catalog entitled, “Isotope Index,” prepared by the Scientific Equipment Co., Indianapolis 19, Ind. Cost of the catalog is $4.
Several companies that prepare and sell standard radioisotopes also will synthesize, on order, less common compounds for special purposes. These companies generally are glad to, advise prospective customers about the preparation, use, and cost of specially synthesized compounds. In addition to the previously mentioned sources of radioisotopes, the AEC, acting through its contractors, Phillips Petroleum Co. (Idaho Falls, Idaho) and Union Carbide Nuclear Co. (Oak Ridge, Tenn.) maintains a service for irradiating suitable metals, compounds, and minerals in high-neutron flux reactors. Special arrangements, however, must be made with the Commission for this irradiation service. Application forms can be obtained by writing to these divisions of the AEC. Several universities and private companies also operate low-neutron-flux research reactors and will contract to irradiate special samples. The cost of the various irradiation services depends on the nature and quantity of the material and the period of irradiation.
The Laboratory Facility
The radiochemical laboratory occupies space formerly used at the Research Center for a foundry and fire assay room. The new laboratory comprises 900
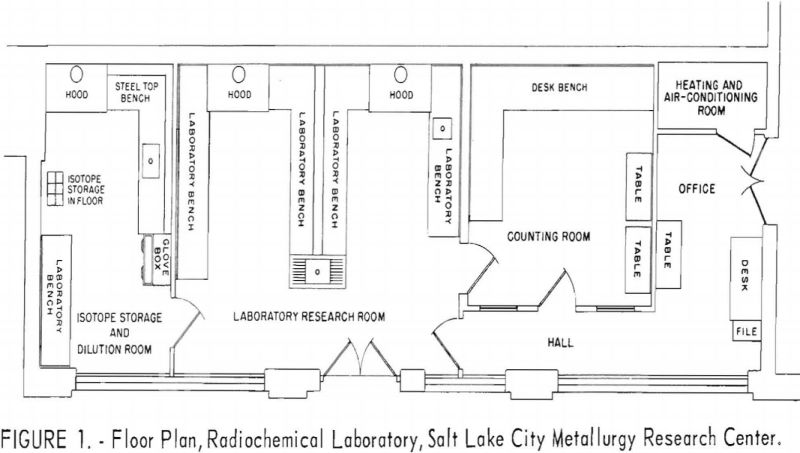
square feet, divided into a small office, a counting room, a low-level-activity research room, and an isotope storage and dilution room. The relationship of the rooms to one another and the placement of the laboratory furniture, benches, and hoods are shown in figure 1. The isotope storage room is used for research involving relatively high levels of activity, which would increase prohibitively the background radioactivity of the counting room if performed in the adjacent low-level laboratory.
Air for the laboratory is drawn from outside the building, conditioned, distributed to the four rooms through ducts in the false ceiling, and finally discharged through the hoods in the low-level and isotope storage rooms. The air in the counting room is maintained at a slightly higher pressure than the air in the low-level and isotope storage rooms in order to minimize the movement of airborne radioactivity into the counting room. The heating, cooling, and air-purification systems for the laboratory are housed in a small closet adjoining the office room. These and the other laboratory utility services are distributed to the work areas via the space above the false ceiling.
The walls of the laboratory are glazed tile, and the windows are glass blocks to facilitate cleaning. The concrete floors are covered with asphalt tile that can be replaced in sections should a radioactive spill contaminate the floor. The research benches are covered with neoprene, and the bench drains are arranged for conveniently monitoring the wastes. The three hoods, one in the isotope storage room and two in the research area, are constructed of stainless steel. Each is provided with external controls for the utility services and contains absolute filters to guard against the discharge of volatilized radioisotopes into the atmosphere.
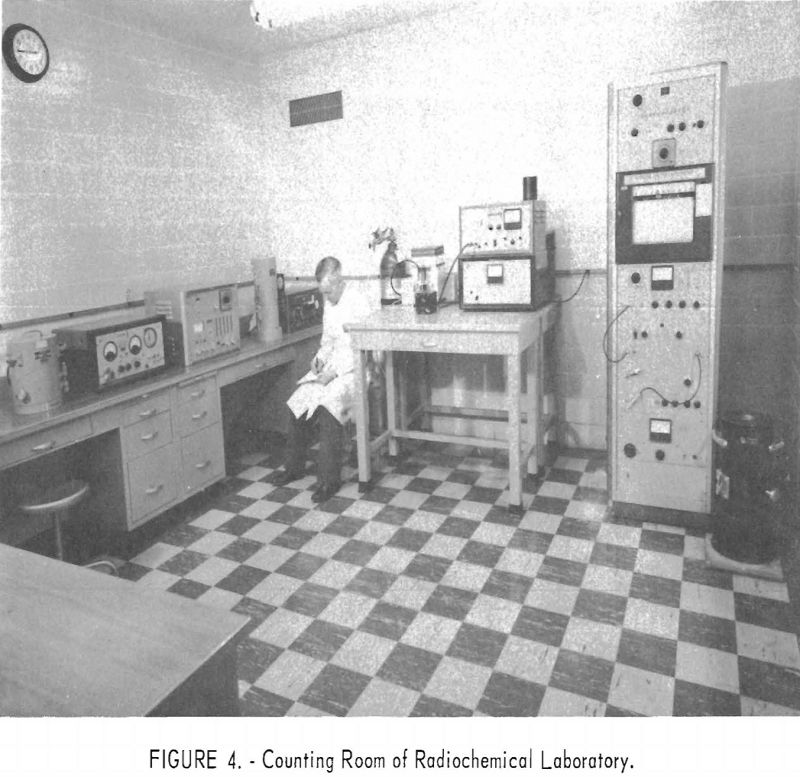
The radioisotope storage vault consists of a block of concrete recessed below floor level. The block contains six compartments, each 12 inches deep and 4.4 inches in diameter. Each compartment contains a steel can that holds a removable sheet-metal basked in which the encapsulated radioisotopes are stored. A square block of lead 2 inches thick serves as a cover shield for each storage hole. Figures 2, 3, and 4 are photographs of the three working rooms.
Instruments and Methods
In addition to conventional equipment such as glassware, filter racks, hotplates, and centrifuges, the laboratory is provided with a glove box, several special tools for remote handling of radioisotopes, a large number of portable lead blocks for shielding purposes, film badges, and other radioactivity monitoring devices. A variety of special instruments are available for identifying and analyzing radioisotopes and for radiometric counting of alpha, beta, and gamma radiations in solutions, gases, and solid products. These include such items as geiger, scintillation, and proportional counters; various types and sizes of fast and slow scalers, ratemeters, and recorders; a variety of shields; several portable laboratory monitoring counters; and a single-channel spectrogammeometer.
Alpha counting is conducted in an internal counter because of the low penetrating power of the alpha particles. Two types of gas-flow proportional counters are available for alpha counting. For routine measurements, a counter having a 2-pi geometry is used. The sample, prepared as a thin, even film on metal foil, is placed in the shallow chamber in the rotatable sample holder. By turning the holder, the sample is advanced from loading to preflush and

then to the counting position. By using a methane atmosphere and a potential of about 2,700 volts, only alpha particles are counted. These weak pulses must be amplified for counting in a scaler having an 0.1-volt input sensitivity. The 2-pi geometry of the instruments limits the sensitivity to a little less than one-half the theoretical count. The laboratory also is equipped with a more sensitive 4-pi counter, which has a lower and upper chamber separated by a mylar film. Experience indicates that this instrument will detect and count about 98 percent of the alpha particles emitted. A zinc sulfide-screen scintillation counter is available when high alpha counting rates are desired.
The more penetrating beta particles are detected and counted in an external counter. For this purpose a conventional geiger tube with a thin mica window is mounted in a 2-inch-thick lead shield with hinged door and plastic shelves. The pulses are counted in a scaler capable of handling approximately 6,000 pulses per minute. Weak beta particles, such as those of C and Ni are counted in the internal flow counters used for alpha counting; however, the potential is raised to 3,850 volts. At this level, the samples for beta counting must be free of alpha emitters, because alpha particles provide a false count considerably higher than normal.
Gamma emitters constitute a large and useful type of isotope that is best detected and counted by scintillation counters. For example, a gamma ray striking a sodium iodide crystal activated with thallium iodide, causes the emission of a light ray, which generates an avalanche of electrons in a photomultiplier tube. The pulses are amplified and counted in a scaler with an 0.1-volt input sensitivity. Liquids and some solid samples are counted in a well-type scintillation crystal. The well accommodates matched glass test tubes or plastic vials, 5/8 of an inch in outside diameter and 5 inches high. Samples to be counted are limited to a 5-milliliter volume to obtain a reproducible geometry and ensure reliable and easily duplicated results. Although scintillation crystals are capable of detecting extremely high decay rates of gamma-emitting isotopes, the conventional binary 64 scaler and 3-decade scalers may be too slow to record the counts. A fast scaler with a 1-microsecond resolving time must be used when the counting rate exceeds 150,000 counts per minute.
The energy of the gamma rays emitted by an isotope is characteristic of that isotope. This unique property of gamma decaying isotopes can be utilized to detect, identify, and quantitatively analyze several isotopes in the same sample. The spectrogammeometer used in this analysis consists of a 2- by 1-¾-inch sodium iodide crystal, mounted with a photomultiplier in a 2-inch-thick iron shield, a power source, linear amplifier, single-channel analyzer, ratemeter, recorder, and high-speed binary scaler. As many as three isotopes with different photopeak energies have been counted and analyzed in the same sample.
Nature and Scope of Research
The radioactive elements and compounds used in the research are chemically and physically like their normal counterpart elements and compounds; however, they have unstable atomic structures that decay when alpha, beta, and gamma rays are released. The emitted rays are readily distinguishable from one another. Alpha particles are helium atoms minus 2 electrons. When a radioactive atom decays by releasing an alpha particle, that atom becomes a new element having an atomic weight of four units less and an atomic number of two less than the parent element. Owing to their large size and relatively low velocity, alpha particles have little power to penetrate solids or liquids. Beta rays are electrons that are emitted at different but relatively high velocities. They have considerably more penetrating power than alpha particles but nevertheless are stopped by a few millimeters of solid materials. The loss of an electron does not significantly change the mass but does increase the positive charge on the nucleus by unity. The new atom advances one valence group in the periodic table, and the new atom is an isobar of its parent. Gamma rays are nonparticulate electromagnetic waves of the same nature as light waves but of much shorter wavelength and therefore of greater penetrating power. Some radioactive substances emit only alpha rays; others, beta and gamma rays; and a few, all three kinds.
The addition of a minute amount of a radioisotope that emits distinctive and easily detectable signals effectively “tags” all the nonradioactive atoms of the same element. These atoms can then be followed accurately through any number of complicated processing steps by simple and rapid radiometric methods.
In this way, fundamental data can be obtained about the nature and rate of many key reactions that would defy analysis because of the complexities of the dynamics involved.
Major attention has been given to the utilization of a variety of radioisotopes in extractive metallurgy and to analytical research on beryllium, cesium, lithium, rubidium, tungsten, scandium, nickel, cobalt, tellurium, and selenium-bearing ores and materials. Intermittent study has been given to employment of the natural radioactivity of uranium- and thorium-bearing ores for monitoring and controlling the operation of a continuous test plant. Some pioneering research also has been undertaken to activate minerals, metals, and compounds in low and high neutron fluxes; to tag specific minerals with selected radioisotopes by chemical and physical absorption methods; and to explore the feasibility of using radioisotopes that emit only weak alpha and beta rays or positrons. Some of the salient findings of the work are summarized in the following sections.
Techniques were devised for monitoring the pressure leaching, countercurrent washing, and solvent extraction operations in the pilot-plant processing of uranium ores. A gamma-sensitive geiger tube and ratemeter were used to sense and control the pulp level in the autoclave by counting the gamma rays emitted from the pulp through the steel shell of the reactor. Similarly, the rise and fall of the liquid-solid interface in the thickeners of the counter-current decantation washing circuit was monitored by immersing beta ray counting tubes in the thickeners. By using a liquid-flow beta-counting tube, the concentration of daughter elements of uranium discarded in the solvent- extraction plant raffinate were determined. A change in the level of radioactivity reflected either overloading or underloading of the solvent-extraction unit.
Preliminary work was done on developing methods for the continuous measurement and control of pulp flow and density in metallurgical operations. Different radioactive sources were tried for beaming gamma rays through pulp streams. The unabsorbed activity served as an index of the changes in the volume and density of pulp flowing past the counting tube. Tests of gamma rays of different energies, led to the discovery that soft gamma rays of intermediate or low penetrating power might be able to solve this problem.
An important accomplishment of the radioactivity research was the development of laboratory techniques, procedures, and counting methods for the effective use of a variety of radioisotopes in hydrometallurgy and pyrometallurgy research. Radioisotopes of beryllium, scandium, cobalt, nickel, iron, aluminum, sodium, selenium, tellurium, rubidium, and cesium were successfully used to study reaction rates, define the effectiveness of alternative processing conditions, and identify trends and anomalies. This research was directed toward providing a guide for the metallurgical-research programs on these elements. Without exception, application of radioactivity techniques simplified and speeded the research and also obviated slow and costly quantitative chemical analysis. Furthermore, as conventional analytical procedures for determining scandium, cesium, rubidium, nickel, and cobalt in the range of a few parts per million were slow and not too reliable, the research on these projects was seriously hampered until isotope dilution techniques were adopted.
Typical Applications of Radioisotopes
A few typical examples will emphasize the unique advantages of using radioisotopes in extractive metallurgy research. The application of Be, Na, Sc, Mn, Co, Zn, Se, Rb, Ag, Sn, Te, Cs, and Hg in solvent extraction, ion exchange, and metallurgical and analytical research concretely illustrates how radioisotopes can be employed to obtain faster and more exact results and to investigate and resolve complex metallurgical and analytical problems difficult or impossible to solve by conventional methods.
A significant saving in time was achieved in research to develop an improved technology for recovering beryllium from low-tenor and complex concentrates by the use of radioactive beryllium. A crucial step in the beryllium extraction process involved the concentration, purification, and separation of the beryllium from a sulfuric acid leach solution contaminated with large amounts of aluminum and iron. By adding a known quantity of radioactive Be to the pregnant leach solution, deportment of all the beryllium was followed through the refining steps by fast radiometric methods. In a typical series of experiments employing solvent extraction techniques for the separation and recovery of beryllium, eight tests were made to determine the loading and stripping characteristics of a particular extractant under different conditions, The tests were completed and the results available to the researcher within 4 hours, whereas 2 or 3 days would have been required had conventional analytical methods been employed. As the results of each test were quickly available, each subsequent test could be better planned to establish trends and define anomalies.
Many available radioisotopes emit detectable rays at an extremely rapid rate. The specific activity of Co for example, is 370,000 disintegrations a second per microgram of the isotope. This high activity of the cobalt isotope has been utilized with impressive results in solvent extraction research to produce cobalt-free nickel from a chloride solution. Prior research on this problem was seriously hampered by the slowness and inadequacy of conventional analytical methods for the determination of cobalt in the range of less than 10 parts cobalt per million parts of nickel. The use of Co as a tracer permitted use of fast radiometric methods for the quantitative determination of as little as 1 part cobalt per million parts of nickel. Indications are that further research could increase the sensitivity to parts per billion.
Extractive metallurgy on many of the uncommon metals and elements heretofore has been hindered by lack of reliable methods for analyzing the raw materials and test products. Using radioisotopes of these elements as tracers and adopting radiometric methods for quantifying and guiding research have eliminated many of the barriers to effective work on the rare and unusual elements. Developing a method for separating, concentrating, and recovering a scandium byproduct from waste solutions derived from processing uranium is a typical problem. Progress was virtually nonexistent until Sc a strong gamma ray emitter, was employed as an easily detectable and measurable tracer to follow the scandium through the several recovery and purification steps.
The use of radioisotopes has facilitated the study of the equilibrium and reaction rates of several complex chemical systems. The application of radio¬isotopes to this kind of problem is exemplified by the use of Na, Rb, and Cs in ion exchange, dialysis, and solvent extraction research to develop a process for separating these alkali elements from one another. The deportment of each of the three elements, tagged with their respective isotope, was readily followed through the various processing steps by determining the characteristic photopeak of the gamma-emitting isotope with a spectrogammeometer.
Radioisotopes can be effectively used in pyrometallurgical as well as hydrometallurgical and mineral-dressing research. The radioactive isotope of selenium, Se was successfully employed in volatilization research on low-tenor ores. Preliminary tests indicated that whereas over 90 percent of the selenium was volatilized from the ore, only 60 percent was accounted for in the test products when using conventional assaying methods. By tagging the ore with a small addition of Se deportment of the selenium in the furnace and in the multiple-stage dust- and fume-collection system was followed accurately by radiometric methods. The “lost” selenium was found as minute deposits of elemental selenium, which had precipitated in cold spots in the furnace and in the ducts connecting the furnace and fume-collection units. All the selenium was located, and the tracer techniques permitted continuous monitoring of the reactions involved to establish the crucial reaction rates.
Investigation of the volatilization and recovery of tellurium from low-tenor gold ores was expedited by employing tracer techniques. The tellurium used as a tracer was prepared from a sample of high-purity tellurium that had been irradiated for 18 hours at a high-neutron flux in the Materials Test Reactor at Arco, Idaho. The irradiated tellurium was dissolved in nitric acid to give a stock solution for storage. A quantitative check showed that the stock solution was far more radioactive than had been anticipated. A spectrogammeometer analysis revealed the presence of resulting from beta decay of Te, which accounted for over 90 percent of the total activity. The radioactive iodine was subsequently removed from the stock solution by distillation. The purified tellurium solution, which contained radioactive Te, Te, Te, and Te, had sufficient activity and half life for about 3 months of use in metallurgical research. A small amount of the mixture of tellurium isotopes, when thoroughly mixed with the tellurium-bearing ore, permitted monitoring of the volatilization experiments to define the reaction rate and to assay the fume, dust, and furnace-residue products with speed and precision.
The use of radiometric methods as a substitute for conventional analytical methods has been mentioned. Experience has shown that radioisotopes and radiometric methods also have many useful applications in analytical research. Sc, Rb, Te and Cs have been used to trace the deportment of these elements through several digestion, precipitation, filtration, and washing steps involved in the quantitative analysis of these elements by chemical, flame photometry, and spectrographs methods. Tracer techniques also have been used in the analytical research undertaken to develop a reliable procedure for the quantitative determination of trace impurities in high-purity tungsten. The method involves the dissolution of a large sample of metal and the precipitation of the dozen or more impurities present as a bulk product.
This precipitate is filtered, washed, and redigested and reprecipitated several times to insure that most of the tungsten is eliminated. The purified bulk precipitate is separated into groups of elements, which can be quantitatively assayed by spectrographic methods. The crucial problem is to insure that all impurities are recovered during the several precipitation, dissolution, and separation steps in the procedure. Radioisotopes of the major impurities expected in the tungsten were added singly or in different combinations to the solution prepared from the metal. Radiometric methods were employed to trace the individual impurity elements and determine their response to different conditions.
Planned Research with Radioisotopes
The radiochemical studies completed to date have probed only a few of the many potential uses of isotopes and “tagged” compounds in extractive-metallurgy research. A few of the more easily used radioisotopes have been employed in metallurgy and analytical chemistry, and limited attention has been given to the possibility of using radioactive source materials for studying the fluid dynamics of mineral pulps. A brief discussion of a few other applications of radioactive materials in metallurgical and chemical research will be presented to conclude this paper.
Not all the elements in the periodic table have radioisotopes that emit easily detectable gamma rays and have a sufficiently long half life to permit their effective use in metallurgical research. The isotopes of many elements emit gamma, beta, and alpha rays, that are relatively weak and difficult to detect. A promising area now under consideration for expanding the use of radioactivity in research is the development of techniques for employing weak alpha, beta, and gamma emitters. Special methods and techniques will be necessary in the research because of the low penetrating power of the rays and the low rate of emission as compared with the strong gamma emitters.
Many problems in mineral dressing, extractive metallurgy, and analytical chemistry may be profitably investigated by radioactivity techniques. The contemplated program at Salt Lake City encompasses basic and applied research in these fields. The chief long-range investigations scheduled include:
- Development of methods to continuously measure and control the pulp flows and densities in mineral-dressing and metallurgical pilot plants using beamed, soft gamma rays.
- Continuation of research directed toward tagging specific minerals with radioisotopes, so that the deportment of these minerals can be accurately followed in milling and metallurgical operations.
- Development of techniques employing radioisotopes and radiometric methods to devise faster and better methods for analyzing trace elements in high-purity metals and quantitatively analyzing rare and uncommon elements.
- Investigation of the feasibility of employing low- and high-neutron and gamma flux reactors for irradiating minerals, metals, and compounds for metallurgical research.
- A study of the effects of radiation and particle bombardment on the rates and mechanisms of metallurgical and chemical reactions. As the different phases of this broad program of research with radioactive techniques in metallurgy are completed, the results will be published.
