Table of Contents
A bastnasite concentrate, containing about 60 percent rare earth oxides, was sulfated with concentrated sulfuric acid to eliminate the carbon dioxide, fluorine, and silica. Calcination at 1,200° F. made the gangue matter insoluble, thus permitting the separation of the soluble rare-earth sulfates. Essentially 100 percent of the rare-earth elements were recovered by this procedure. Since the rare-earth sulfate solution contained only trace amounts of non rare-earth elements, subsequent separation procedures were simplified.
An efficient and economical chemical procedure was used to achieve desired separations of the rare-earth group. The rare-earth sulfates were precipitated with caustic, followed by air-drying at 120° C. to oxidize the cerium to the tetravalent state. The trivalent rare-earth elements were acid-leached, leaving a residue of fairly pure cerium. The final step was the removal of most of the lanthanum, which was accomplished by monitoring the neodymium with a rapid-scanning spectrophotometer during precipitation of the remaining rare-earth elements with ammonia.
Ion-exchange separations were made on the cerium-free rare-earth elements, using sulfonated polystyrene-divinyl benzene resins and ethylene diamine tetra-acetic acid (EDTA) as an eluant. The effects of resin particle size, resin cross linkage, and eluant flow rate were determined. With resins of small particle size and intermediate porosity, relatively high flow rates were used without decreasing the efficiency of separation. Several divalent metals were added to the feed-stock solutions to determine their elution order with the rare-earth elements. It was possible by this technique to effect a complete partition of samarium from neodymium and cerium from lanthanum. The major conclusion from these ion-exchange studies was that individual rare-earth elements of any desired purity could be produced by proper selection of operating conditions.
Solvent-extraction techniques were also used for separating cerium from the other members of the rare-earth group recovered from bastnasite. Cerium was converted to the tetravalent state by electrolytic oxidation, extracted with tri-n-butyl phosphate (TBP) from a nitric acid solution containing the rare-earth elements, and recovered from the organic phase with dilute hydrogen peroxide. In small-scale tests, using separatory funnels, it was possible to extract 95 percent of the cerium at 99+ percent purity. Cerium extractions were also accomplished using a pressure-bubbler-type system. Cerium recoveries of about 85 percent at 99+ percent purity were achieved. This closed-circuit extraction system permitted better control of operating variables, and could be adapted to large-scale processing of rare-earth elements.
Introduction
In recent years interest in developing uses for the rare-earth metals and compounds has grown.. Also, more attention has been given to the strategic importance of this group of metals. Because of the extensive interest, and in anticipation of an increasing need, the Bureau is conducting research to develop efficient and economical methods of concentrating, separating, and purifying the rare-earth elements.
The designation rare earths is misleading, owing to the relative abundance of this group of elements, which are found in various minerals such as monazite, bastnasite, euxenite, xenotime, and gadolinite. It is not the scarcity of the rare-earth elements but rather the marked chemical similarity that is the main obstacle to research and development. Of the rare-earth minerals available, bastnasite was selected for this particular investigation to take advantage of two important factors:
- The supply of bastnasite is ample to permit expansion of domestic rare-earth element production. There is an extensive high-grade deposit near Mountain Pass, Calif.
- Bastnasite is a plentiful source of the lighter or cerium rare-earth elements.
Historically, Scandinavia may well be described as the ancestral home of the rare-earth minerals, with the first rare-earth mineral being discovered at Ytterby, Sweden, in 1788. Bastnasite apparently derived its name from having been discovered near Bastnas, Sweden. Bastnasite is commonly designated by the formula (RE)FCO3 where RE signifies the rare-earth elements.
The mineral contains about 75 percent rare-earth elements, the major constituents being cerium, lanthanum, minor amounts of neodymium, praseodymium, and samarium.
The literature indicates that only limited research efforts have been directed specifically toward concentrating and separating the bastnasite rare-earth elements. Zadra and coworkers have described ore-dressing methods used on bastnasite. Kirk and Othmer presented a comprehensive technology of rare-earth elements that included a brief commentary on the opening of bastnasite ores with acids. Spencer, Yost and coworkers, and Vickery have published textbooks on the chemistry of the rare-earth metals. Separating adjacent rare-earth elements by ion exchange was developed concurrently, but independently, during World War II at Ames Laboratory, Ames, Iowa, and Oak Ridge National Laboratory, Oak Ridge, Tenn. Both laboratories were leaders in ion exchange and solvent-extraction techniques.
This report discusses the development and/or adaptation of suitable methods for the separation and purification of the bastnasite rare-earth elements. Techniques were devised employing a combination of methods—classical chemical, ion exchange, and solvent extraction-to permit efficient processing of bastnasite concentrate to obtain the individual rare-earth elements.
Source of Bastnasite Concentrate
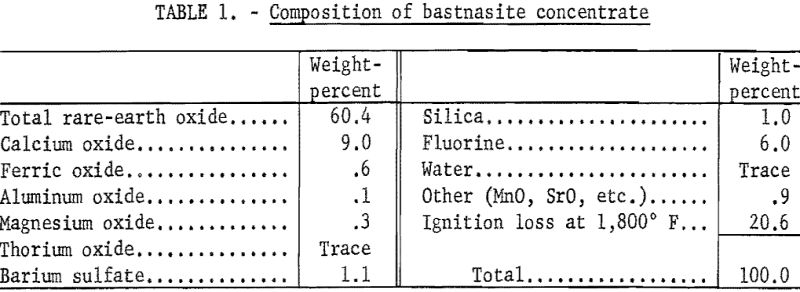
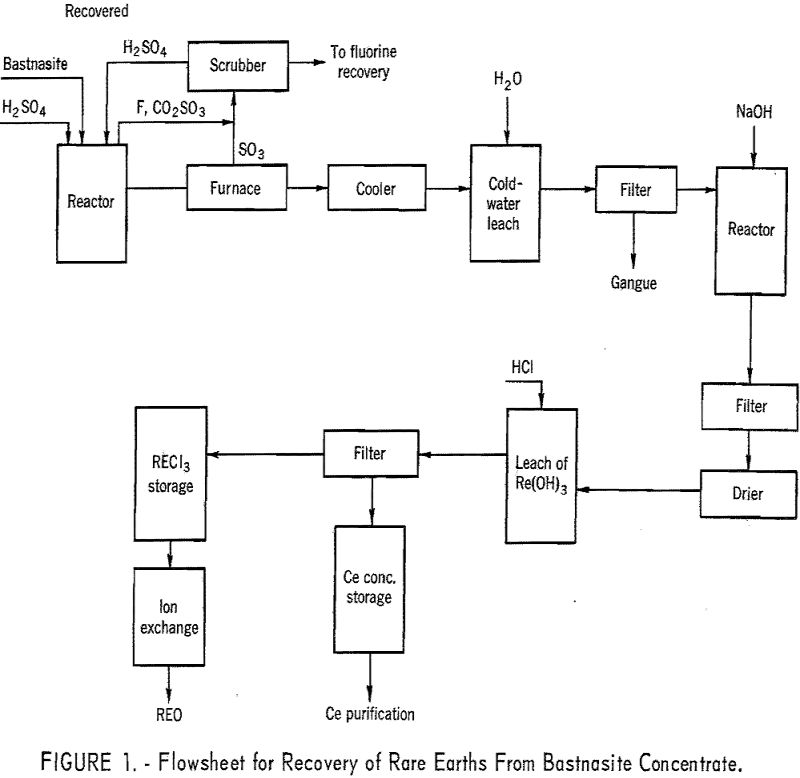
A bastnasite concentrate containing 60 percent rare-earth oxides was obtained from the Molybdenum Corporation of America plant at Mountain Pass, Calif. A flotation method had been used to produce the concentrate from a bastnasite ore containing approximately 10 percent total rare-earth oxides. This company also produces a 72-percent concentrate by a countercurrent decantation leach of the 60-percent flotation concentrate with a 10-percent HCl solution, and a 90-percent concentrate by calcining the 72-percent concentrate at 1,000° F. to remove carbon dioxide. A chemical analysis of the 60-percent concentrate is shown in table 1. An X-ray-emission spectrographs analysis of the rare-earth elements from the 60-percent concentrate is shown in table 2. The X-ray-analysis technique was based upon comparing the test samples with synthetic standards approximating the composition of the test sample.
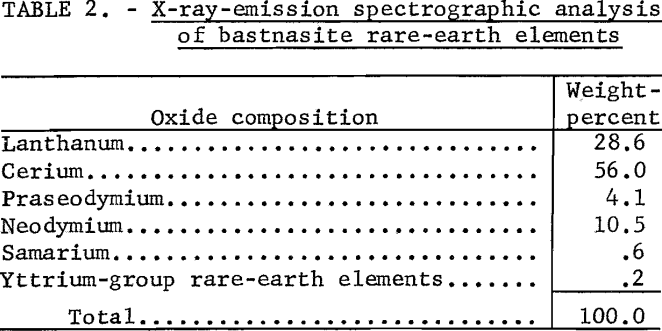
Chemical Processing of Bastnasite Concentrate
Initial studies for processing bastnasite concentrate consisted of treating the calcined concentrate with dilute acids, employing the sodium hydroxide method that is sometimes used in treating monazite ores and treating the concentrate directly with concentrated mineral acids. The studies are described briefly below. Bastnasite ores have been processed using dilute acids in a technique similar to the sulfuric acid process for monazite.
After a preliminary calcination at 1,000° F. to remove the carbon dioxide, the bastnasite concentrate was treated directly with dilute acids to dissolve the rare-earth elements. However, the efficiency of rare-earth recovery was not high because of the formation of an insoluble rare-earth fluoride, which was difficult to handle and contaminated with unreacted gangue material.
The sodium hydroxide method consisted of reacting the ore with a hot concentrated aqueous solution of sodium hydroxide to convert the rare-earth phosphates to hydroxies, followed by the dissolution of the hydroxides with hydrochloric acid and the ultimate precipitation of a high-grade rare-earth hydroxide product by neutralizing the chloride solution. However, when this method was applied to the bastnasite concentrate, the fluorine was only partially removed, making it necessary to treat the hydroxide material with concentrated sulfuric acid for complete removal of fluorine and recovery of the rare-earth sulfates.
Preliminary extraction tests were also made on the concentrate with concentrated mineral acids (sulfuric, hydrochloric, and nitric). Of the three acids used, only the sulfuric acid removed the fluorine and permitted good recovery of the rare-earth sulfates.
The processing technique adopted as a result of these studies is shown in figure 1. The concentrate is added to warm concentrated sulfuric acid in the ratio of 1 pound of concentrate to 1.3 pounds of acid. The reaction is exothermic and is controlled by the rate of addition of the concentrate to prevent the reaction from becoming violent. After frothing ceases, the temperature is raised to 900° F. and kept constant for 4 hours. The fluorine, carbon dioxide, and silica are completely eliminated at the end of the 4-hour reaction time.
After the sulfation is complete, the dry granular sulfates are calcined for 2 hours at 1,200° F. to render the gangue constituents insoluble to water. Most of the gangue constituents form water-insoluble oxides, except the calcium sulfate, which forms the insoluble anhydrite. At lower calcination temperatures the gangue remains partially soluble in water.
The water-soluble rare-earth sulfates are leached with cold water and separated from the gangue by filtration. Although the amount of rare-earth sulfates contained in solution is not critical, a supersaturated solution should be avoided. Rare-earth sulfates are more soluble in cold than hot water. A desirable solution and one that stores well for subsequent treatment contains approximately 50 grams of RE2O3 per liter,
Chemical Separation of Rare-Earth Elements from Bastnasite
The usual procedure for separating the various rare-earth elements from one another in sulfate solutions is by oxalate or double-sulfate precipitation. Both precipitation techniques involve a number of time-consuming steps and the use of relatively expensive chemicals. A technique was used whereby the rare-earth elements were precipitated directly with sodium hydroxide. The key to the success of this procedure depended on a sulfate solution, obtained by leaching the calcined concentrate that contained essentially only rare-earth metals.
Cerium, the major constituent, was separated from the other elements of the rare-earth group by the method of Powell, in which the hydroxides were dried at 120° C., utilizing air oxidation to convert cerium to the tetravalent state. The trivalent elements then were dissolved in dilute hydrochloric acid at 60° C., leaving a cerium oxide residue. The solubility of the cerium was increased by leaching at elevated temperatures and by lowering the pH below 2.8. The progress of the leaching step was monitored by analyzing the solution for neodymium with a Beckman DU spectrophotometer, and the addition of acid was stopped when 95 percent of the neodymium was in solution. The results of the chemical treatment are summarized in table 3.
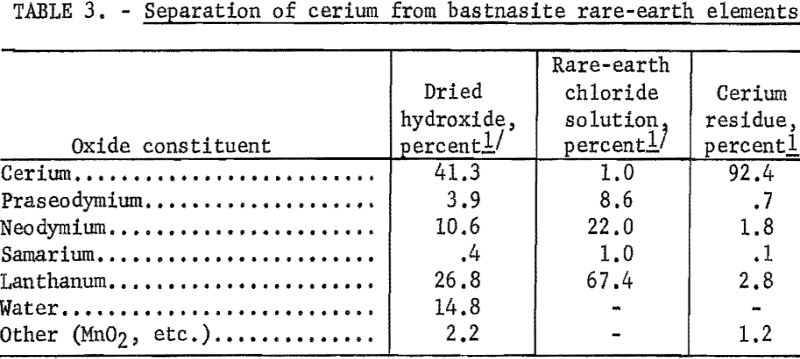
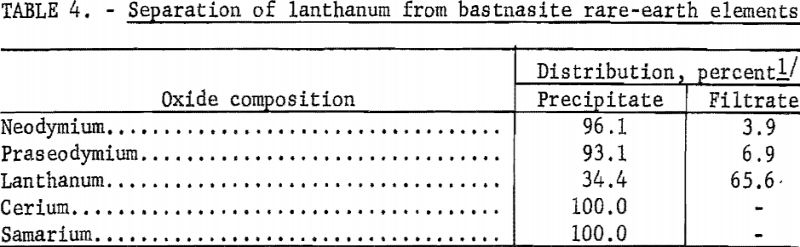
Although the rare-earth chloride solution could be used as a feed stock for ion-exchange columns, it was desirable to enrich the feed as much as possible with the less abundant rare-earth elements by prior chemical processing. A technique was developed for chemically separating the lanthanum, which comprised about 67 percent of the rare-earth chloride solution. Spencer suggested that a lanthanum separation could be effected by controlling the pH while precipitating with dilute ammonium hydroxide, as neodymium, praseodymium, and samarium are precipitated at a pH of 6-7, while lanthanum is not precipitated below pH 8. In the procedure established, the decrease of neodymium and praseodymium in the solution was observed with an American Optical Company rapid-scanning spectrophotometer. Dilute hydroxide was added until the minimums these two elements caused in the absorption curve disappeared.
In the preparation of a feed stock for ion exchange, two consecutive hydroxide precipitations were performed, and table 4 gives typical results for this separation technique. These data show that about 65 percent of the lanthanum was left in solution while essentially all the other rare-earth elements were precipitated.
The precipitate was treated with hydrochloric acid to give a rare-earth chloride solution suitable for ion-exchange processing.
In summary, the method developed for chemically separating the rare-earth elements contained in bastnasite consisted of precipitating the elements with caustic, separating the trivalent rare-earth elements from cerium by acid-leaching, and removing lanthanum by controlled precipitation with ammonia. Further separation of the remaining elements by chemical means was not considered feasible. The method has the advantages that the chemical procedure involved is quite simple, and that the equipment and chemicals required are relatively inexpensive. In addition to providing an enriched rare-earth concentrate, the method simultaneously provides comparatively pure cerium and lanthanum. Thus the chemical processing is highly important to provide the desired feed stocks for further separation by solvent extraction and ion exchange.
Ion-Exchange Separations
The use of ion-exchange techniques for separating bastnasite rare-earth elements was investigated, since this appeared to be the only efficient process for producing individual elements of high purity. Previous work used EDTA for elution and resin in the divalent copper cycle for band development.
The studies consisted of experiments to determine the effects on degree of separation obtained by varying resin particle size, resin cross linkage, and eluant flow rate, and by adding zinc, cobalt, iron, and manganese to feed-stock solutions. A typical analysis of the ion-exchange feed stock was as follows: Lanthanum oxide, 20 percent; cerium oxide, 2 percent; cerium oxide, 2 percent; praseodymium oxide, 22 percent; neodymium oxide, 50 percent; samarium oxide, 5 percent; and other rare-earth oxides, 1 percent.
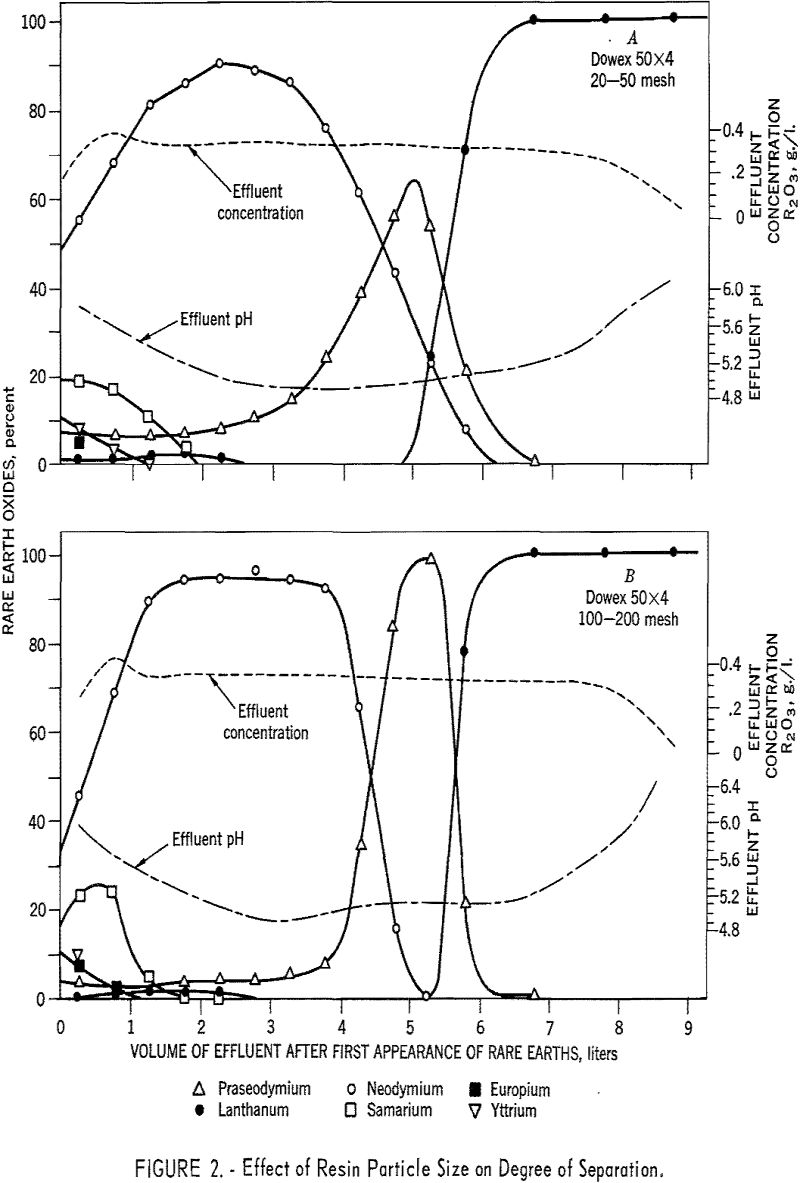
Resin particle size was found to have a marked effect on degree of separation. Figure 2 shows graphically the effects of eluting 3.0 grams of rare earths through the remainder of 1.5- by 92-cm. ammonia-state beds of 20- to 50-mesh and 100- to 200-mesh Dowex 50 x 4 resin with 0.005 molar EDTA at a pH of 7.0 and flow rate of 2.0 ml./min./sq.cm. With the 100- to 200-mesh resin, not only were the element purities much higher, but, of more importance, higher yields of maximum purity were obtained. The increases in degree of separation were attributed to the increase in surface area and the shorter distance the rare-earth complexes had to diffuse to reach the sulfonic groups in the resin matrix.
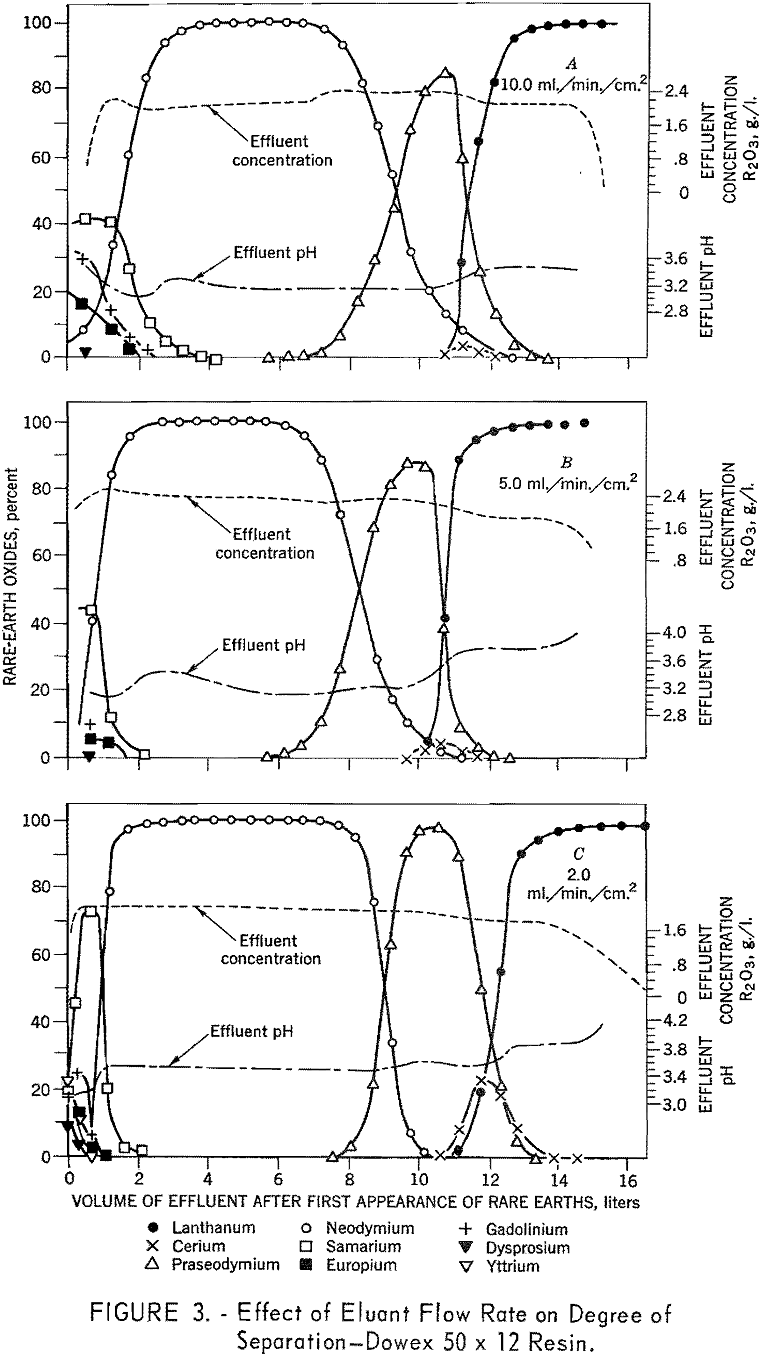
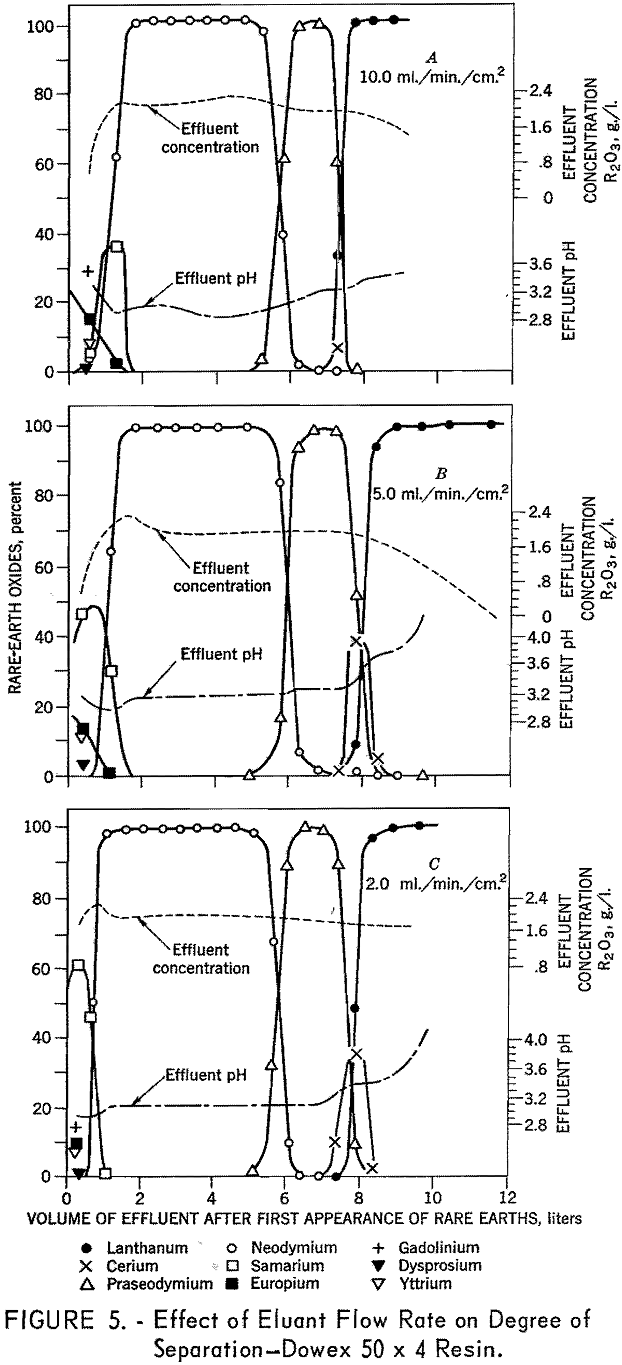
To determine the effects of eluant flow rate on degree of separation, six 1.7- by 104-cm. columns containing 100- to 200-mesh Dowex 50 x 12 resin were arranged in sets of two for three separate runs. In each test, the first bed was saturated with rare-earth elements and the second with divalent copper, the rare-earth elements were eluted from the first column of each pair through the second column of each pair in the copper cycle with 0.015 molar EDTA adjusted to a pH of 8.0. A flow rate of 10 ml./min./ sq.cm, was used in the first three runs, then 5 ml./ min./sq.cm., and finally 2 m./min./ sq.cm. Duplicate tests were made using 100- to 200-mesh Dowex 50 x 8 and x 4 resins. The data presented in figures 3, 4, and 5 demonstrate the effects of flow rate with various resins on separation of the rare-earth elements. The 8- and 12-percent divinyl benzene (medium and low porosity) resins gave favorable separations at low flow
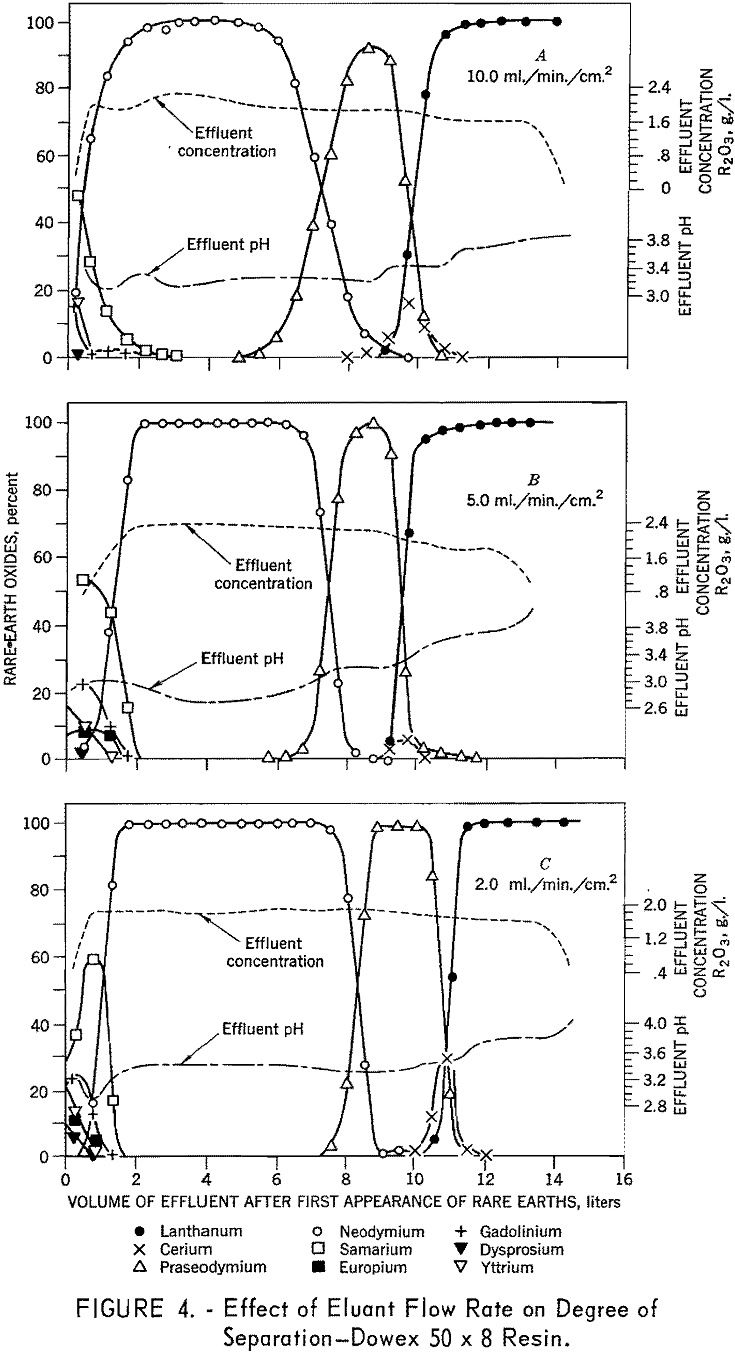
rates and poor separations at higher flow rates. In contrast, the 4-percent divinyl benzene (high porosity) resins gave favorable separations at all flow rates. The decline in separating efficiency with increasing divinyl benzene content and flow rate is attributed to the decrease in porosity which inhibits diffusion in and out of the resin matrix. The tests using 4-percent divinyl benzene resins yielded approximately 82 percent of the neodymium with a purity in excess of 99 percent. In similar tests, using a flow rate of 0.3 ml./ min./sq.c.m., 97 percent of the neodymium was recovered with a purity in excess of 99 percent.
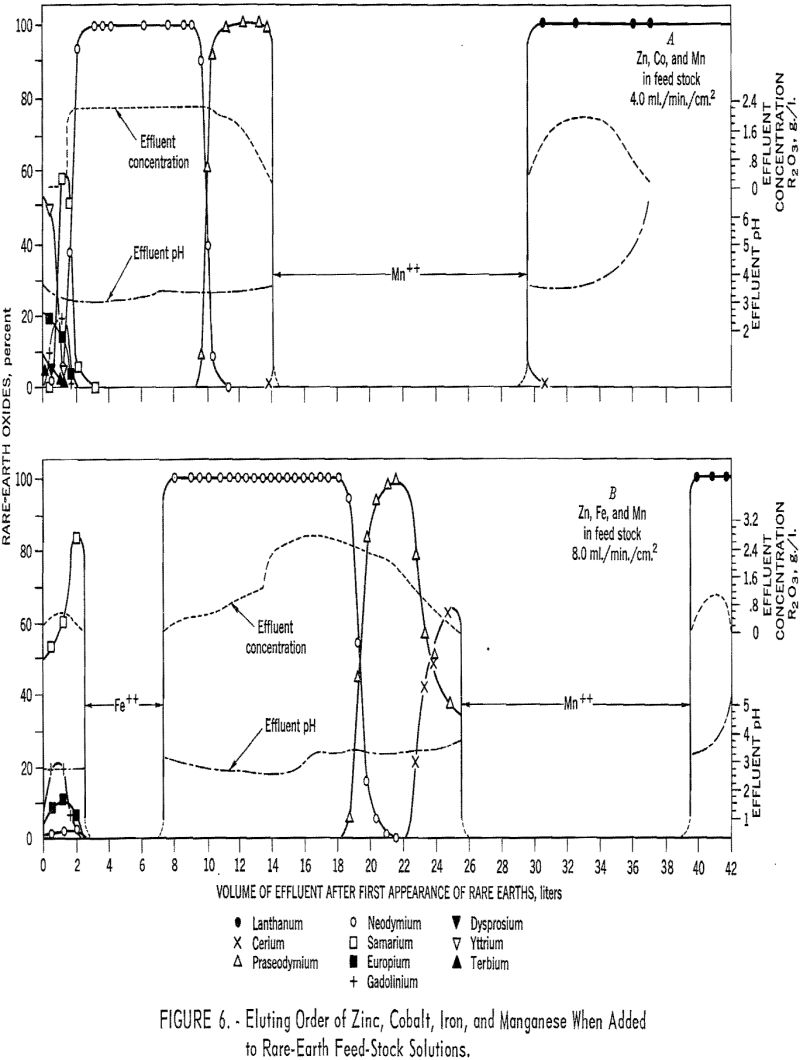
Divalent zinc, iron, cobalt, and manganese were added to ion-exchange feedstock solutions to determine their elution order with the rare-earth elements and effects on separation. To obtain the data presented in figure 6, 40 grams of rare-earth elements and 20 grams each of zinc, cobalt, and manganese were eluted from two 3.2- by 78-cm. columns of
16- to 20-mesh IRC-120 resins in the copper II cycle and one 2.2- by 127-cm. column of 100- to 200-mesh Dowex 50 x 4 resin in the copper II cycle. EDTA with a molarity of 0.015 and pH of 8,0 was used as the eluant, and the flow rate was 4.0 m./min./sq .cm, based on the 2.2-cm, column. For the data shown in figure 6, the conditions were identical except that iron was substituted for the cobalt, and the eluant flow rate was 8.0 ml/min./sq.cm. Zinc and cobalt eluted with the samarium and heavier rare-earth elements, and iron eluted between samarium and neodymium, which resulted in a complete partition of these elements. The manganese eluted between cerium and lanthanum in both cases.
These data show that ion-exchange methods can be adapted for the separation of rare-earth elements from bastnasite. With a 4-percent divinyl benzene (high porosity) resin of 100- to 200- mesh particle size and flow rate of 2 to 10 ml./min./sq. cm,, individual elements of 99+ percent purity can be recovered. If complete partition of samarium from neodymium, and cerium from lanthanum, is desired, divalent iron and manganese can be used.
Therefore, the desired purity of the individual rare-earth elements will govern the choice of operating conditions for ion-exchange processing. With
a series of larger columns and the proper operating conditions, it would be possible to separate the major portion of each of the predominant rare earths in bastnasite in a single pass. The heavier elements present in small quantities would be collected until a sufficient amount was accumulated to warrant a separate run.
Solvent-Extraction Separations
Solvent-extraction techniques and equipment for processing the bastnasite sulfate product also were considered. Since cerium constituted more than 50 percent of the rare-earth elements in this material, the initial work was directed to its separation.
Other workers have utilized solvent extraction for isolating cerium. Wylie has shown that eerie nitrate in 5-6 M nitric acid is readily extracted by diethyl ether. Bock and Mayer report extraction of 99-percent-pure cerium at 85-percent recovery with diethyl ether. Spectrographically pure cerium also was produced by Klinaev and Senyavin by using diethyl ether. The use of tri-n-butyl phosphate (TBP) for cerium extraction was studied by Warf. In a three-stage crosscurrent extraction, he recovered more than 99 percent of the cerium from a pure cerium nitrate solution.
TBP was chosen for this investigation in preference to diethyl ether because of its greater resistance to oxidation, insolubility in acid solutions, and lower vapor pressure. This choice was made despite the fact that diethyl ether is more selective for cerium (IV).
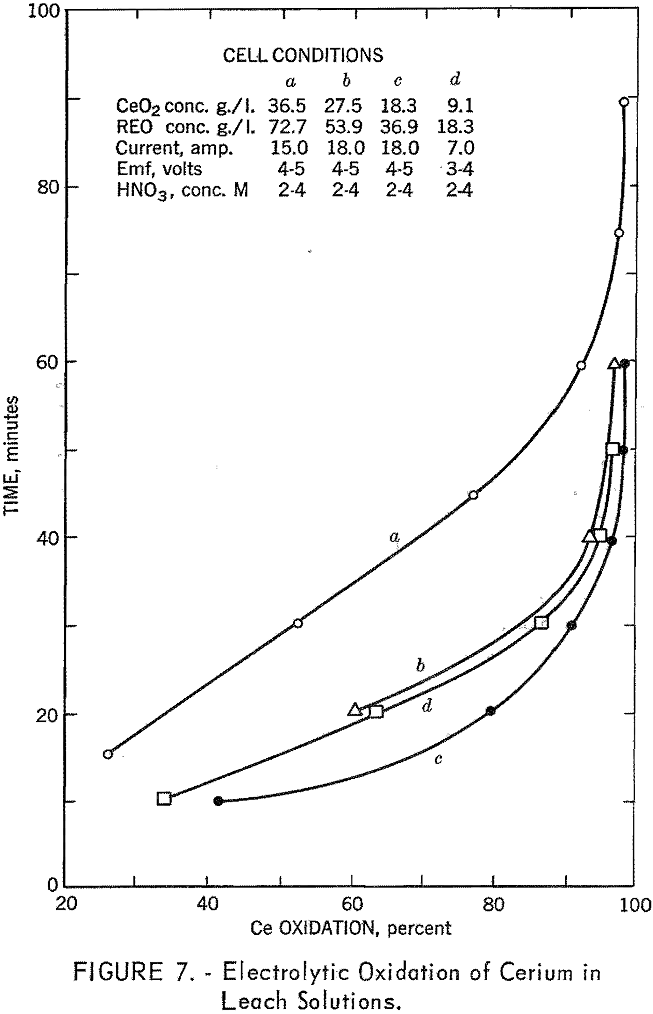
In order to extract the cerium efficiently, it was necessary that it be in the tetravalent state. Cerium can be oxidized chemically, but this adds to the solution another metal that must be subsequently separated. It was decided to oxidize the cerium electrolytically, as this added nothing to contaminate the raffinate. Up to about 90 percent of the cerium could be oxidized by this method, as shown in figure 7.
In the extraction procedure adopted, the anhydrous sulfates of the calcined bastnasite were water-leached to produce a solution of approximately 50 grams of rare-earth oxides per liter. This solution, after filtering, was made 2 M with nitric acid and then oxidized electrolytically. After the acid content had been adjusted with nitric acid, this solution was contacted with TBP, either pure or diluted with benzene. The cerium was stripped from the organic phase with dilute hydrogen peroxide solutions.
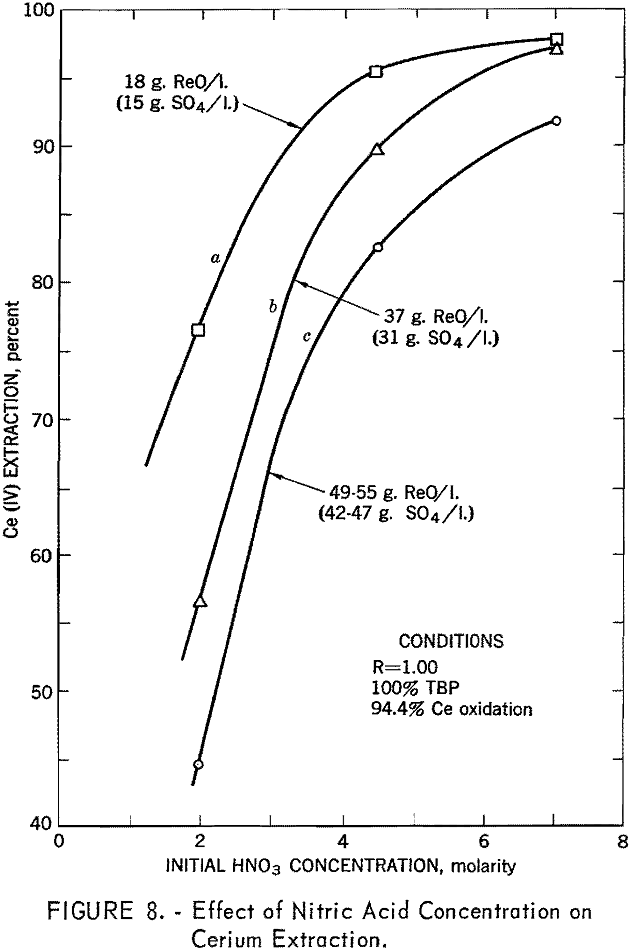
Of the variables investigated, the phase ratio and the concentrations of TBP, nitric acid, and sulfate ion were considered to be of the greatest importance. Nitric acid concentration in the feed was the most important controlling factor in tetravalent cerium extraction. Cerium extraction increased with increased nitric acid concentration up to a maximum of 6 to 8 M, depending on rare-earth and sulfate-ion concentration (fig. 8). There was, however, a tendency for cerium purity to decrease at the higher acid strength, owing to greater transfer of trivalent rare-earth elements.
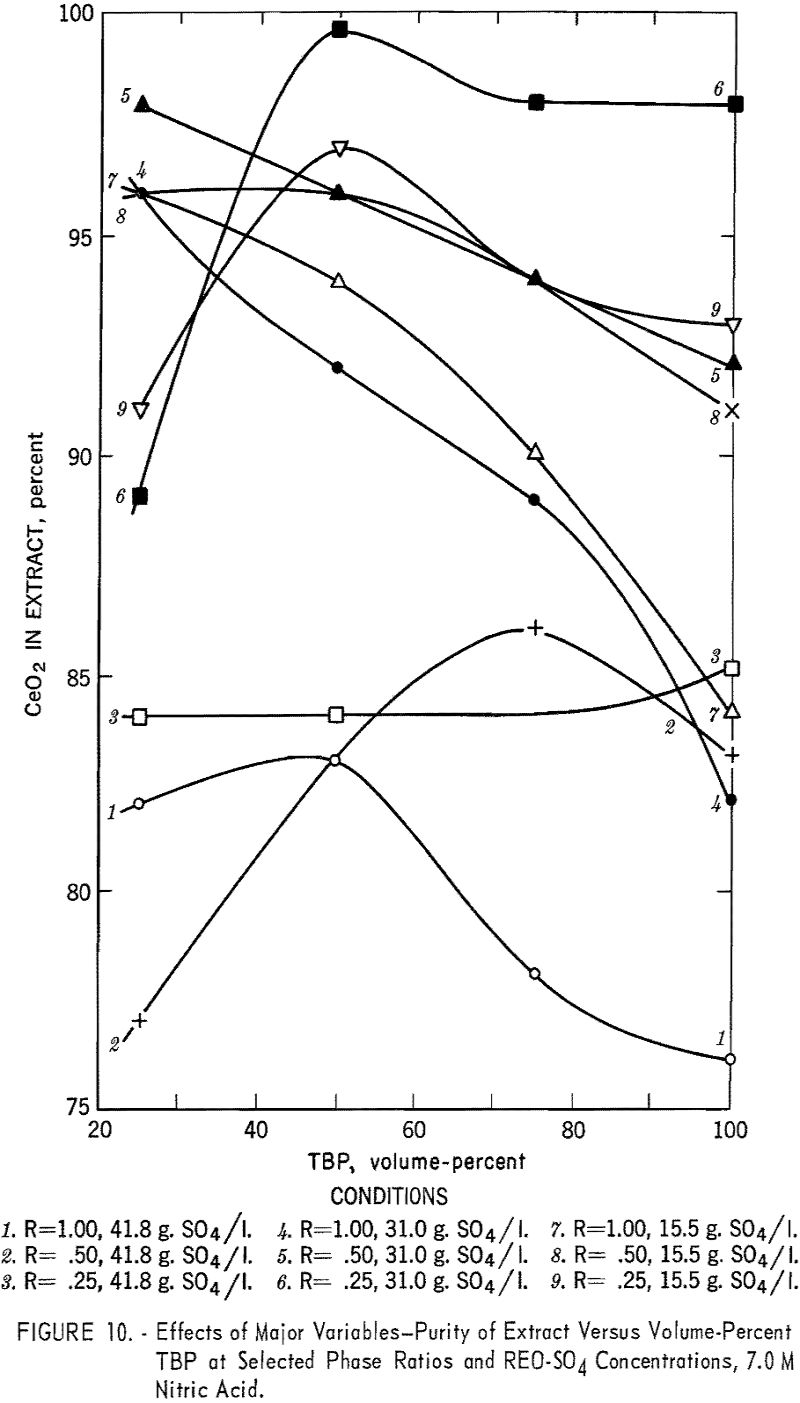
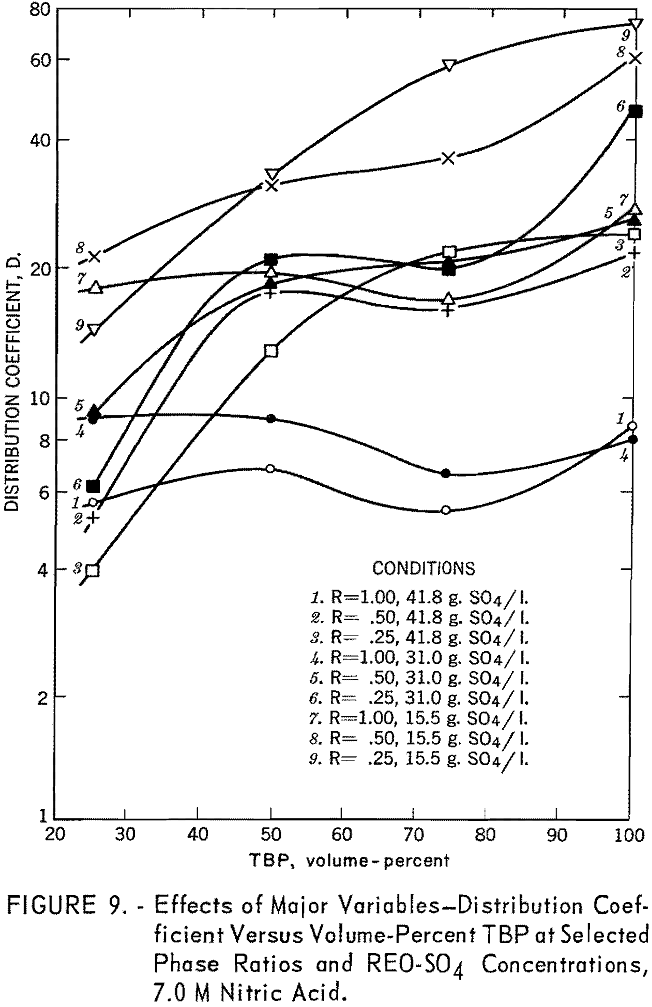
The effects of phase ratio and TBP concentration were intimately connected. Generally, an increase in TBP concentration resulted in increased cerium extraction, as illustrated in figure 9. The effect of phase ratio varied with the TBP concentration; at higher TBP concentrations a lower phase ratio produced better results, while at low TBP concentrations the effect was the opposite. Their combined effect on cerium purity was somewhat more complex. In most cases cerium purity was highest at low phase ratios and TBP concentrations of 40 to 70 percent (fig. 10).
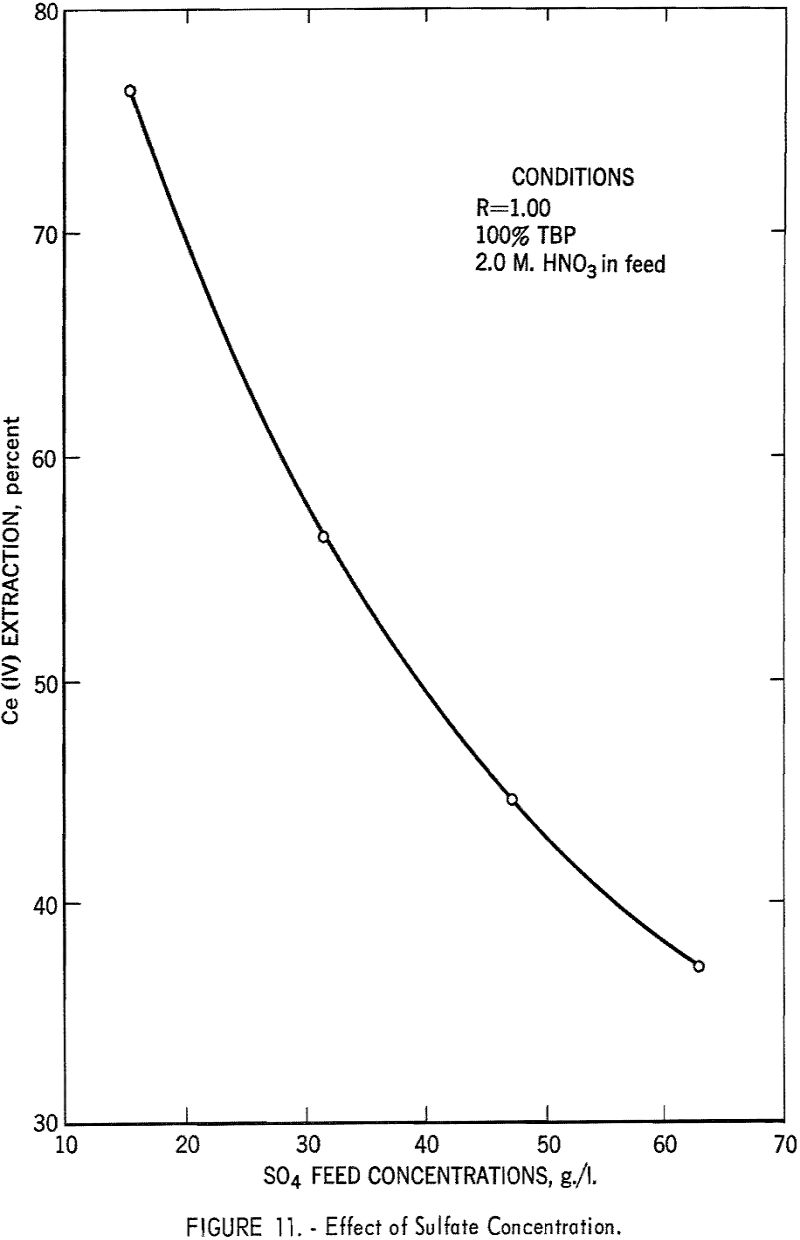
The effect of sulfate ion is shown in figures 9, 10, and 11. As shown in figure 8, this effect can be largely overcome by high nitric acid concentration; however, when sulfate-ion concentration exceeds 40 grams per liter, cerium extraction decreases sharply.
In summary, this phase of the investigation showed that by using TBP concentrations of 40 to 60 percent, low phase ratios in the order of 0.25, and nitric acid concentration in the feed of 6 to 8 M, and by keeping the sulfate ion concentration below 40 grams per liter, efficient extraction of cerium could be attained. In small-scale tests it was possible to extract 95 percent of the cerium at 99+ percent purity in a single stage.
A study was also made of pressure-bubbler-type equipment for carrying out the cerium extraction. Mass transfer in this type of equipment is subject to precise control, which depends on flow rates, column size, and bubble size. It was possible, therefore, to vary the relative mass-transfer rates in successive columns of the system over a wide range. In most tests, the feed was prepared as previously described, while the organic phase consisted of equal amounts of TBP and benzene. The organic phase (discontinuous phase) was recycled directly back to the feed container, thus forming a closed circuit. A typical flow schedule for the organic phase was to bubble through the following containers in sequence;
- Feed container. Picks up cerium (IV), nitric acid, and a small amount of trivalent rare-earth elements.
- Water scrubber. Drops off almost all of the trivalent rare-earth elements along with most of the nitric acid.
- Sodium sulfate scrubber. Cerium (IV) is removed along with most of the remaining nitric acid.
- Acidifying container. Picks up nitric acid before returning to the feed container.
Difficulties were sometimes encountered because the organic and aqueous phases approached the same specific gravity value. This was especially prone to happen in the water scrubber. The most serious consequence was for the aqueous phase to be transferred out of the water scrubber with the organic phase, lowering the purity of the cerium extract. Although means of preventing this situation have not received much attention to date, it probably can be handled by controlling the percent TBP in the organic phase and the concentration of rare-earth elements in the feed. For this investigation, the problem was alleviated by incorporating a secondary calming section after the water scrubber. This piece of equipment was essentially a glass column packed with glass helices.
The cerium was recovered from the back extraction solution by pH adjustment and the addition of oxalic acid. The rare-earth oxalates were filtered, washed, and converted to the oxide. The
precipitation was carried out in this manner mainly for analytical purposes. In actual practice, the cerium would be recovered by hydroxide precipitation or possibly by precipitation as the simple basic, or double sulfate.
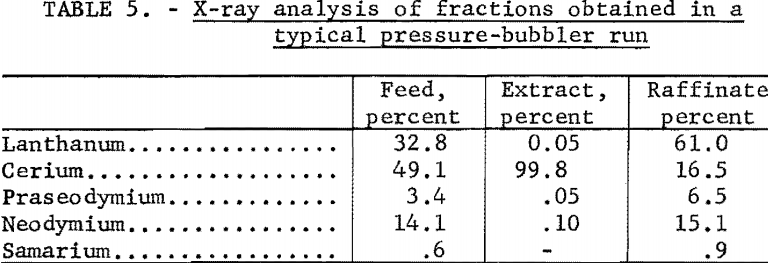
Cerium recoveries exceeding 85 percent at 99+ percent purity have been achieved. Table 5 shows a Typical X-ray analysis of the extract, raffinate, and feed. Work in progress is aimed at increasing the recovery to a minimum of 95 percent. Also, the possibility of converting the system to continuous operation is being considered.
The use of TBP for extracting and separating tri-valent rare-earth elements has not received much attention; however, Scardill and coworkers have achieved separation factors as high as 2 for successive rare-earth elements by TBP extraction. Therefore, it is possible that by expanding such research, liquid-liquid solvent extraction might become an integral step in rare-earth separation and purification.