Table of Contents
Although there are many potential ceramic uses for kaolinitic materials of the type occurring in the Eufaula district, this investigation has been limited to a study of their refractory properties.
Sampling was restricted to deposits uncovered by bauxite-mining operations, and no effort was made to determine continuity of clay strata from pit to pit. Rather, it was hoped to obtain the widest possible range in composition, since mine operators estimate that clay reserves total more than 1 million tons in the Baker Hill area alone.
Samples were collected from seams that had been previously exposed in bauxite-mining operations. The clays were sampled at frequent intervals along the exposure, and a minimum total weight of 100 pounds was taken from each deposit. Clay strata averaged 10 feet in thickness and were covered by varying depths of sandy overburden, generally less than 20 feet.
Fourteen locations were represented in the sampling. Five samples originated in the northwestern corner of T. 8 N., R. 28 E., Henry County; the remainder in T. 9 N., R. 27 E., Barbour County, Ala.
It was possible to make a rough distinction between bauxite, bauxitic kaolin, and kaolin in place by differences in texture. The kaolins were fine-grained, dense, waxy to the touch, and light buff. (The bauxitic kaolins were compact but they did not exhibit the smooth texture and lustre of the purer clays.) The bauxites were pisolitic in nature, friable, and cream to white.
Preparation of Samples
Field samples were collected as crude lumps that contained about 15 percent mechanical water. After drying overnight at 110° C. the samples were crushed to pass a 6-mesh material after each pass through the crusher.
Head samples of 1,000 grams were riffled from each crushed clay to provide material for preliminary test work.
Description and Identification of Samples
Screen Analyses
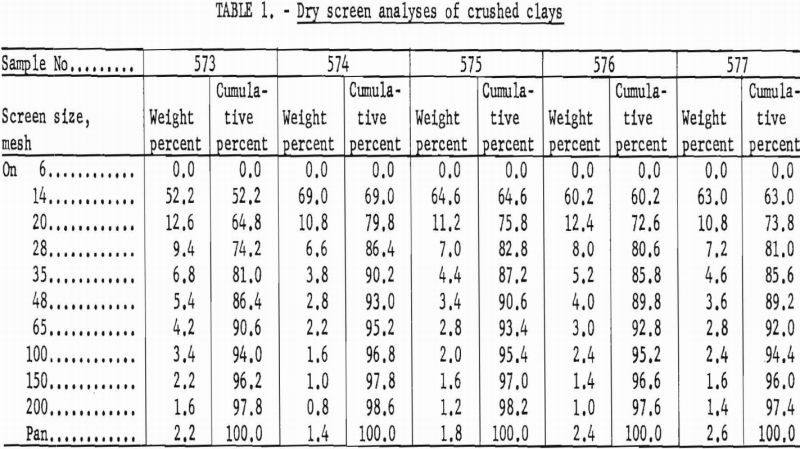
Crushing characteristics of the clays were determined by dry screen analysis of 500-gram samples using Tyler Standard screens in a Ro-Tap set for 15 minutes.
The size gradations produced by preliminary grinding were considered satisfactory in all samples. More than 50 percent of the material was retained on a 14-mesh screen while the amount of fines was nominal. Typical screen analyses are shown in table 1.
Chemical Analyses
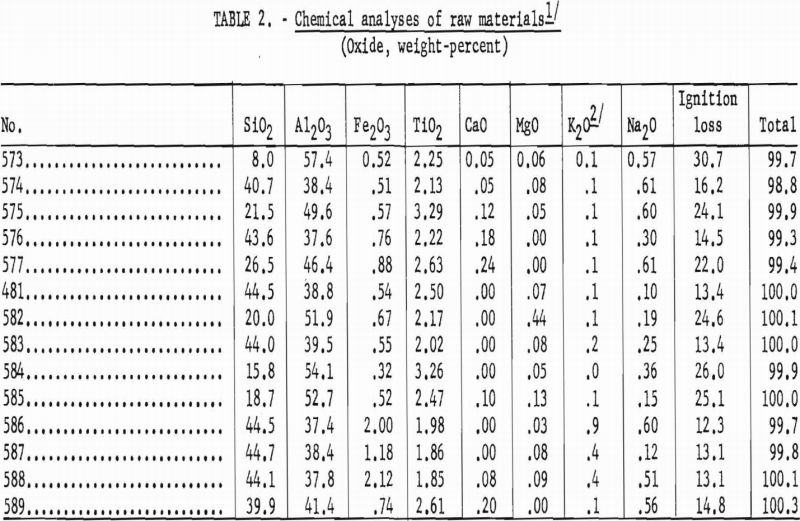
Chemical analyses provided a basis for ore classification and indicated the range of alumina content in the kaolins as sampled.
Data in table 2 show the kaolins to be quite uniform in chemical composition and similar to the commercial kaolins of Florida.
The bauxitic kaolins exhibited a wider range in chemical composition than that shown by the kaolins. The variation was due mostly to differences in the kaolin-bauxite ratio that were reflected in the silica contents.
Mineral Composition
Approximate mineral composition of the samples was determined from X-ray diffraction patterns, chemical analyses, differential thermal analyses, and petrographic determinations.
Typical differential thermal analysis curves are illustrated in figures 2 and 3. Endothermic peaks near 360° C. are characteristic of gibbsite, while
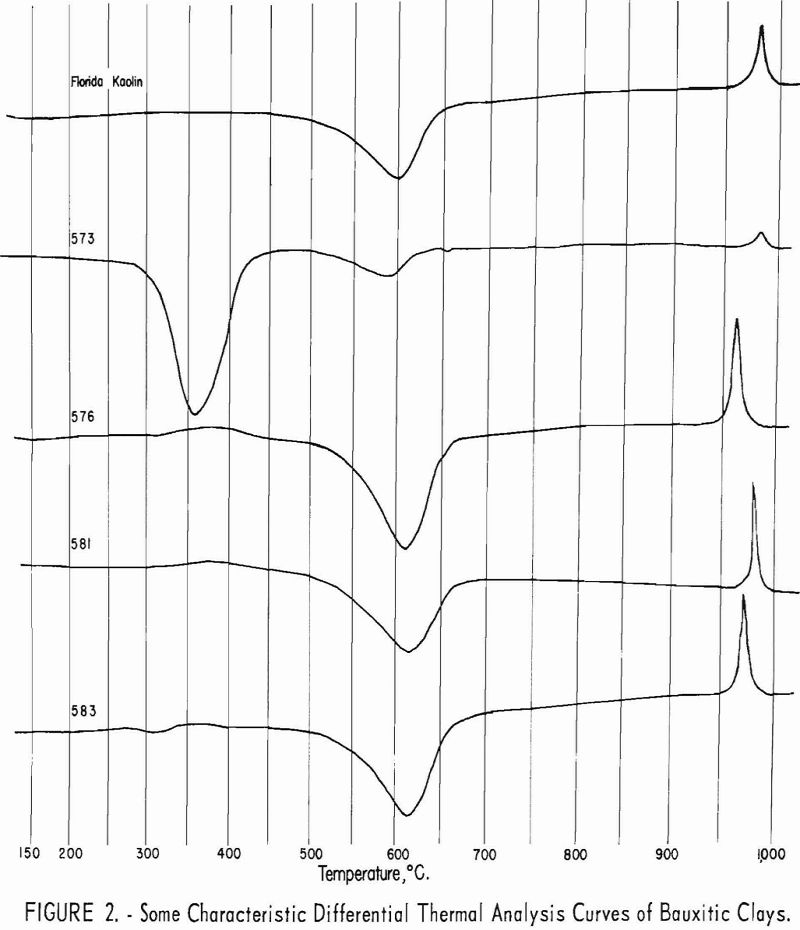
endothermic peaks near 625° C. and exothermic peaks near 980° C. are characteristic of kaolinite.
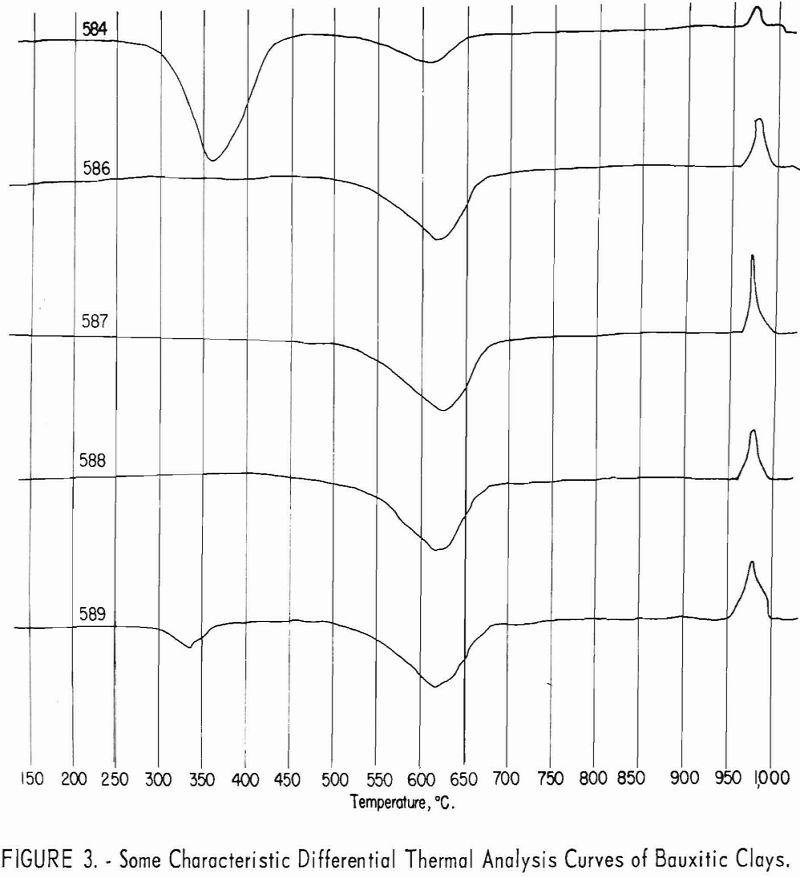
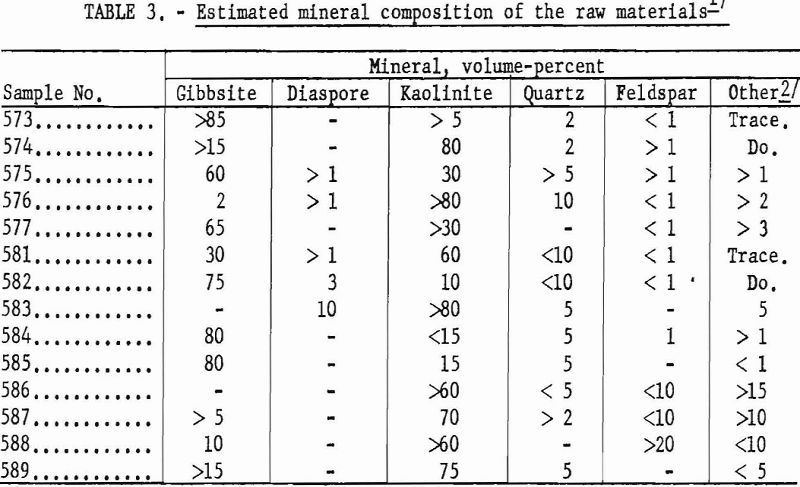
The ores are mixtures of kaolinite and gibbsite, accompanied by minor amounts of quartz, mica, and feldspathic glass. The minerals indicated by X-ray diffraction patterns to be dominant in each of the samples are listed in table 3.
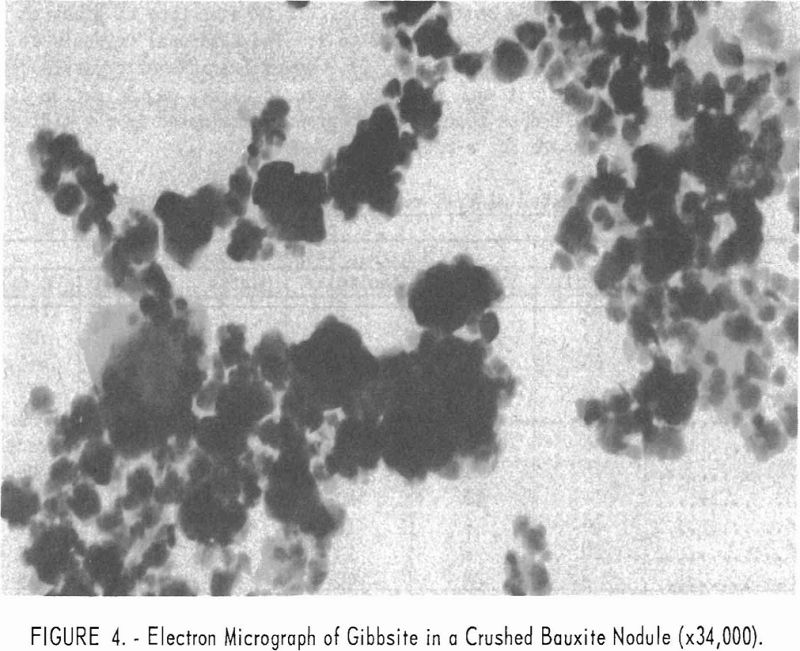
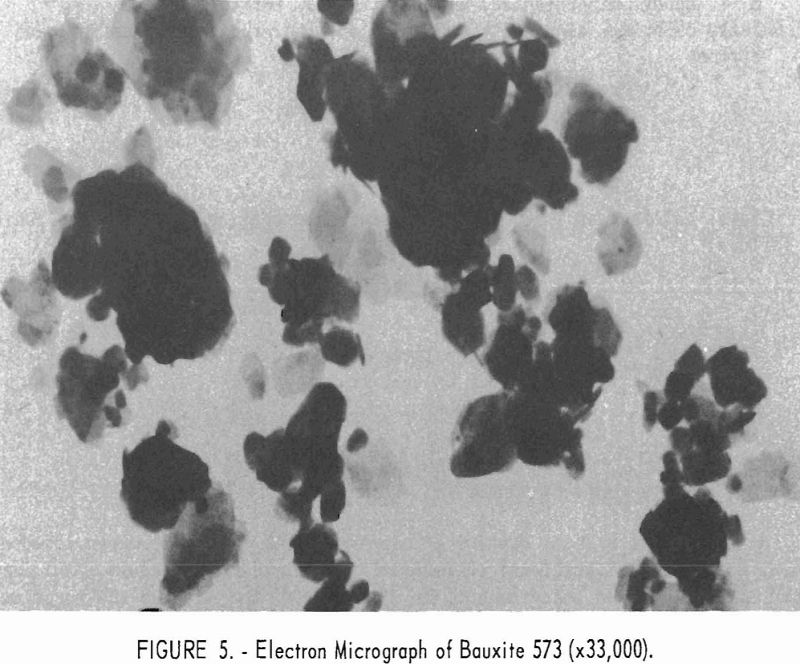
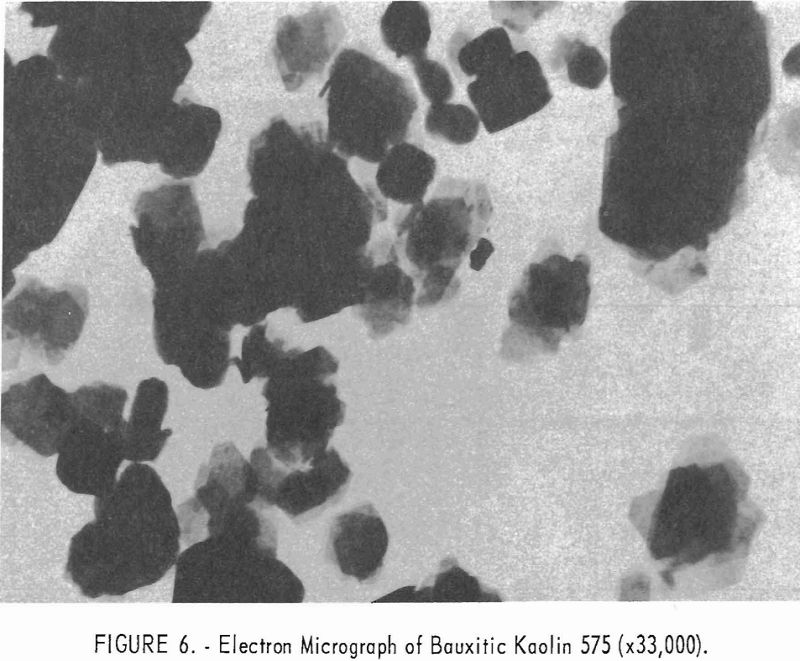
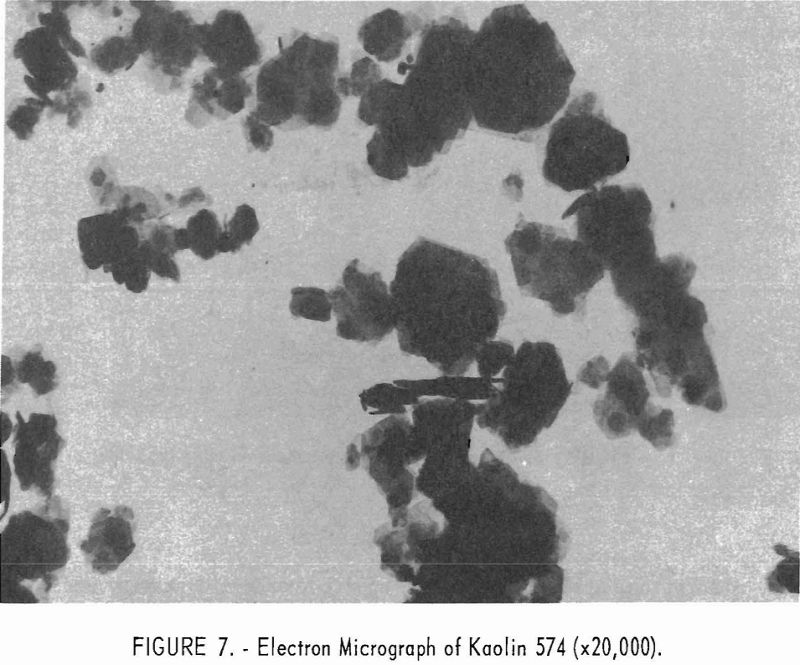
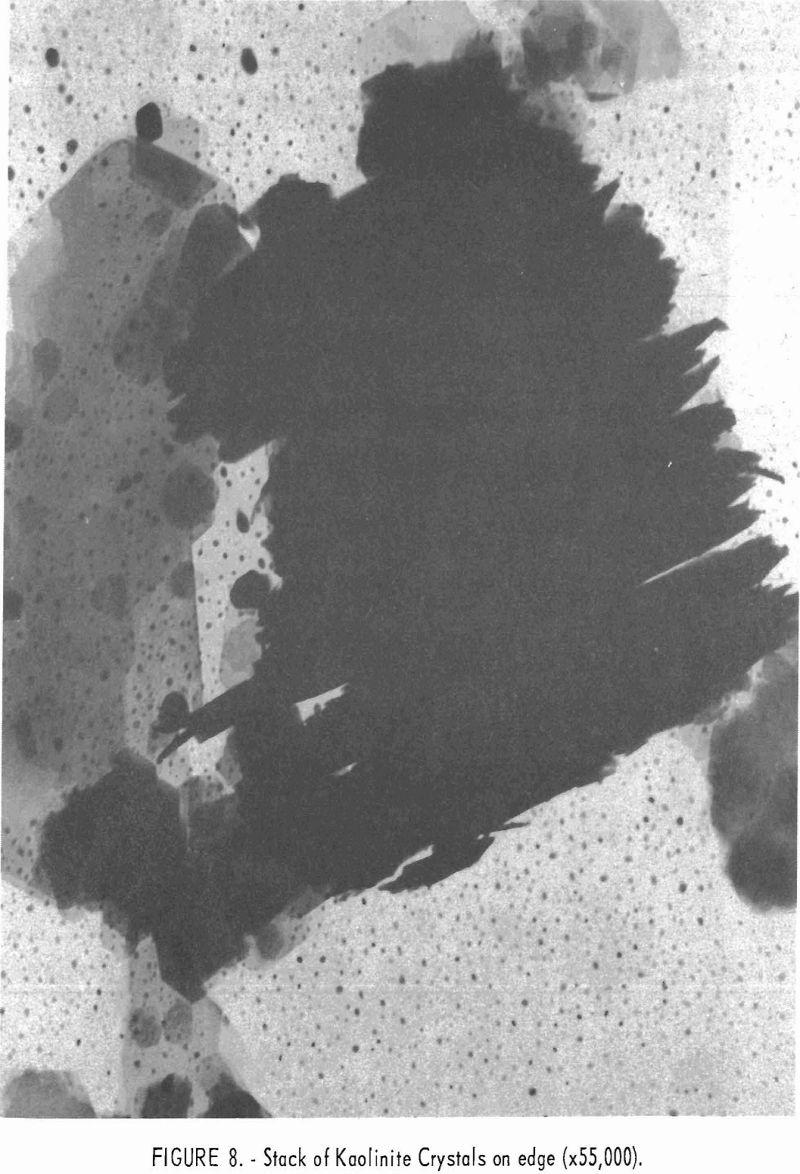
Electron micrographs (figs. 4, 5, 6, 7, and 8) illustrate the range in mineral composition of the samples.
Gibbsite in a crushed bauxite nodule is pictured in figure 4. Flattened prisms of the monoclinic gibbsite are quite irregular in size and generally much smaller than the hexagonal kaolinite crystal that appears in the lower left of the photograph.
Mullite-grade bauxite 573 is shown in figure 5. Gibbsite prisms pre-dominate, with occasional kaolinite crystals that account for most of the silica content (7.6 percent).
Sample 575 (fig. 6) is a bauxitic kaolin. It is a mixture of gibbsite and kaolinite in an approximate ratio of 2 to 1. The hexagonal crystals of kaolinite predominate in sample 574 (fig. 7). Several stacks of crystals on edge are in evidence, and one such stack is shown at higher magnification in figure 8. The many small dots forming a background originated in the medium used for suspension and are not a part of the sample.
Dry-Press Refractories
Preparation and Firing
Clays, calcined at 1,300° C., were used in preparing the dry-press bodies as follows:
Mixtures were blended dry for 10 minutes, about 5 percent water added and mixing continued an additional 10 minutes. Trials were pressed at 8,000 p.s.i., dried at 110° C., and fired to cone 18 down (1,490° C.) with no soaking time.
Physical Testing
Absorption, apparent porosity, apparent specific gravity and bulk density were determined on 2- by 1- by ¾-inch briquets. The average of the values obtained with five specimens is reported for each property.
Pyrometric Cone Equivalent
Preliminary refractory classification of the raw materials was based upon fusion points determined by comparison with standard pyrometric cones fired in an oxygen-acetylene furnace. Raw clays were ground to minus-65-mesh, calcined at 980° C., and formed into cones using gum arabic binder.
Compressive Strength
Crushing strengths were determined on 2- by 1- by ¾-inch briquets using a hydraulic testing machine with speed of the moving head set at 0.04 inch per minute. Pieces were tested on end using cardboard caps to equalize surface irregularities. The average of the values obtained with five specimens was reported.
Modulus of Rupture and Fired Shrinkage
Modulus of rupture was determined on 4- by 3/8- by 3/8-inch bars, using a 3-inch span between bearing edges. Fired-shrinkage figures were also obtained from these bars. The average of the values obtained with five specimens was reported.
Reheat Change
The permanent linear change upon reheating was determined using 3-½- by 1-½- by 1-¼-inch miniature brick. Specimens were set in a bed of alumina and fired to 1,400° C. in 3 hours. After soaking 5 hours at 1,400° C. the test pieces were allowed to cool at the natural kiln rate of approximately 100° C. per hour. The average of the values obtained with three specimens was reported.
Refractoriness Under Load
Deformation under load at high temperatures was measured on 3-½- by 1-½- by 1-¼-inch brick in a maintained temperature test with dead load of 50 p.s.i. The gas-fired furnace reached 1,450° C. in 1-¼ hours, and this temperature was maintained 1 hour. Subsidence was measured on the Ames dial, calibrated to 0.001-inch.
Thermal Shock
Resistance to thermal shock was determined, using 3-½- by 1-½- by 1-¼-inch brick. An electric kiln was preheated to the required temperature with dummy brick in the wicket. Trial brick were then inserted and heated for 10 minutes, removed, and immersed in tap water to a depth of 1-½-inch for 2 minutes, followed by steaming on a steel plate for 8 minutes. This cycle was repeated until the test pieces could be broken between the fingers – indicating failure.
Thermal Expansion
The coefficient of thermal expansion was determined by using 2- by 3/8- by 3/8-inch bars. Measurements were made in a platinum-wound furnace in which a temperature rise of 3.5° C. per minute from 20° to 1,200° C. was maintained. Synthetic sapphire rods supported a dial gage that indicated the differential in expansion between supports and specimen. The dilatometer was calibrated, using a bubble-free, fused-silica reference rod.
Results of Physical Testing
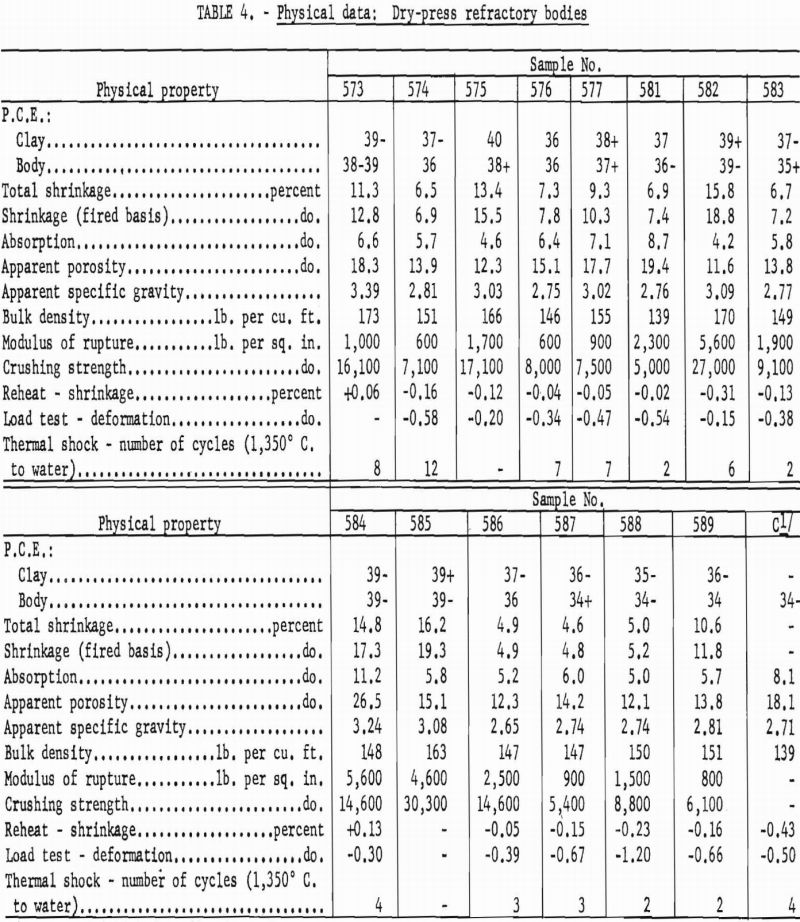
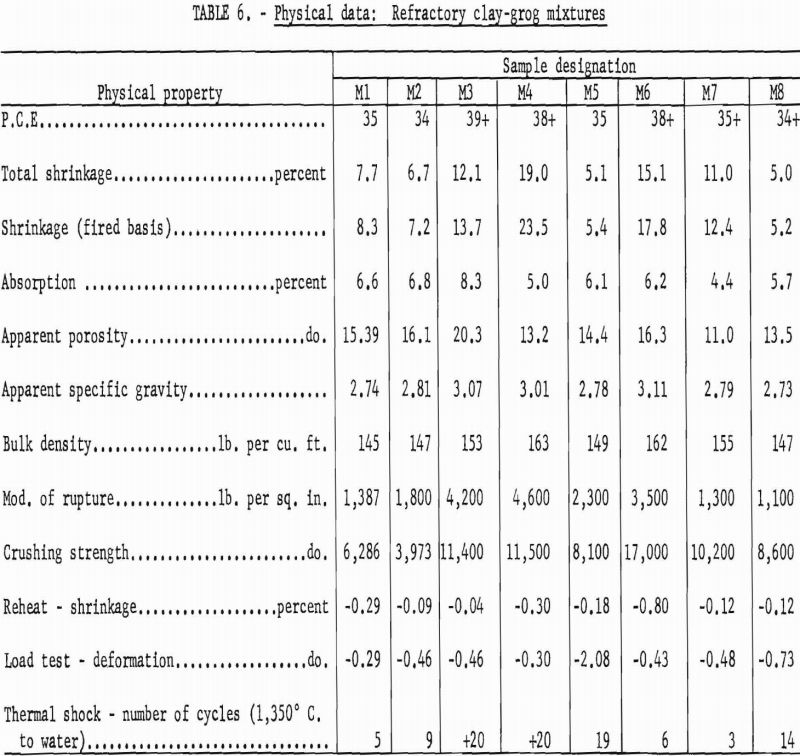
Materials that have a pyrometric cone equivalent (P.C.E.) of not less than cone 21-½ in the fired material, a modulus of rupture in excess of 1,200 p.s.i., and a minimum bulk density of 137 lb. per cu. ft. are classed as high duty slag resistant refractories (ASTM Designation: C-27-56). Physical properties of bauxitic kaolins, when processed as refractory bodies, meet the specifications for high-duty service and compare favorably with commercial fire-brick of like class. Test data are summarized in table 4.
Although the softening points of all compositions were about the requirement for superduty refractories (cone 33, 1,745° C.), preliminary trials processed at 1,600° C. were overfired and obviously unsatisfactory for use in the simulated service tests required of this class.
Kaolin 588 showed much higher subsidence in the hot load test than others of the group. This weakness can be attributed to the presence of 15-20 percent of feldspathic glass having an index of refraction approximating that of an albite-type feldspar.
A high gibbsite content tended to Increase both the softening point and the shrinkage of test bodies. Grog-calcination temperatures higher than the 1,300° C. used in this work would be essential to provide shrinkage control in this type mixture.
Clay Blends
Preparation and Testing
It is often desirable to blend raw materials from several sources, not only to minimize natural physical variations but to obtain the most desirable combination of fired properties. Several mixtures were studied, using one clay as grog and another as the bond. The composition of each blend is shown in table 5. Preparation and testing followed the procedures outlined in the previous section.
Results of Blending Tests
Test results are summarized in table 6. In general, physical properties of the fired mixtures reflect those of grog, as it is the predominant component.
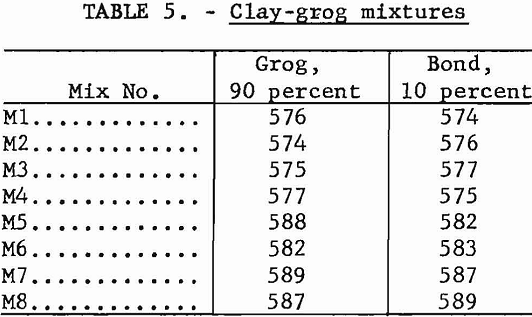
Mixtures M4 and M6 were high in gibbsite; this accounted for the excessive shrinkage and fusion points above cone 38 (1,835° C.). Mixture M5 failed in the high-temperature load test, again demonstrating the weakness shown by kaolin sample 588 in previous tests.
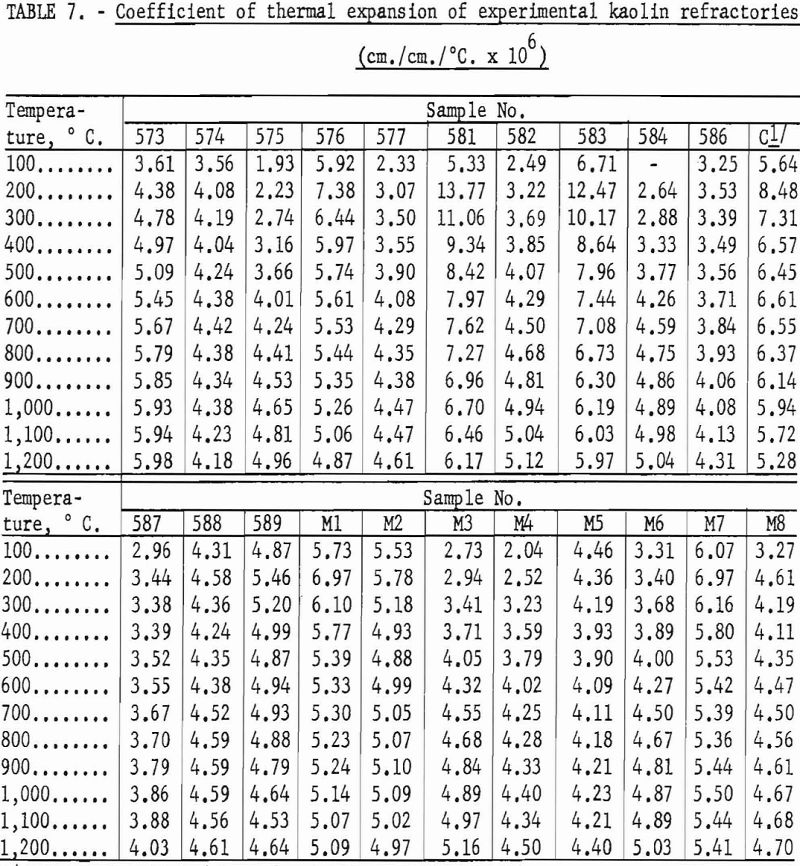
Fireclay refractories that have a high degree of mullite crystallization are usually characterized by a comparatively low coefficient of thermal expansion and good resistance to thermal shock. Based on data in table 7, it may be assumed that the experimental refractories equal the commercial product in resistance to spalling action.
In general, mixtures high in gibbsite showed the greater thermal expansion figures, and mixtures high in kaolinite the lower figures. A cristobalite inversion is apparent in the 200° and 300° figures for samples 576, 581, and 583. Kaolin 588 and mixture M5 containing cristobalite show insignificant variation throughout the test range, which is another indication of the glass content that must have caused weakness in the high-temperature load test.
Refractory Specialties
Specialty products are an important segment of the refractories industry. Products so classed include the mortars used for laying firebrick, the castables used in boiler construction, and the plastic firebrick used for monolithic furnace linings, incinerators, and boilers.
Air-Set Mortar
Air-set mortars are designed for smooth application in thin joints which have good dry strength and yet will not flow out at service temperatures. After necessary adjustments were made to provide for desired working qualities a satisfactory mortar was prepared as follows:
The mortar was tested for refractoriness by constructing a pier consisting of a full 3-½- by 1-½- by 1-¼-inch briquet, two half pieces , and another full piece, the set providing two horizontal joints and one vertical joint, each 1/8 inch thick. The pier was air-dried 24 hours, dried at 110° C. overnight, and then fired on the superduty schedule (ASTM Designation: C113-46) maintaining the required 1,600° C. for 5 hours.
Strong, sound joints were formed by both the test mix and the commercial mortar fired for comparison. No shrinkage, peeling, cracking, or flowing from the joints was in evidence.
Refractory Castable
When tempered with water, castable refractories develop structural strength due to hydraulic set. An experimental castable was prepared as follows :

Test bars were hand-tamped in a 7- by 1-¼- by 1-inch brass mold, care being taken to form homogeneous specimens. Bars were dried at 110° C. and fired to 1,400° C. in 3 hours, with a 5-hour soak. Physical properties of the experimental and a commercial castable are shown in table 8.
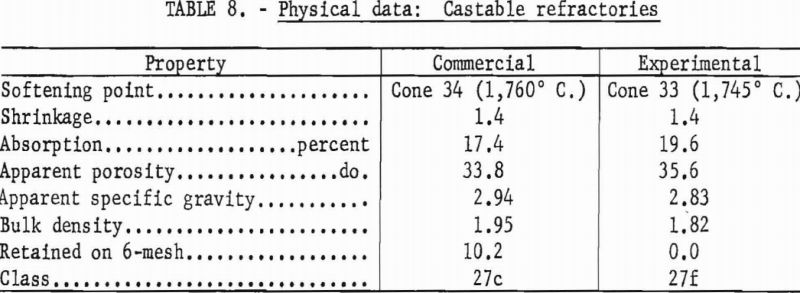
The experimental castable meets specifications of class 27f (ASTM Designation: C213-51), wherein not more than 5 percent of the dry material may be retained on a 6-mesh sieve, and the permanent linear change must not exceed ± 1.5 percent after heating at 1,400° C. Because more than 5 percent of the dry material was retained on a 6-mesh sieve the commercial castable is placed in class 27c.
Plastic Refractories
Plastic refractories are used for the monolithic construction favored in many furnace installations. Being supplied as a moldable, the material must carry enough water to assure good bonding when rammed, yet it must not be so mobile that it slumps when forms are removed, and shrinkage must be nominal. The following experimental plastic refractory was prepared:

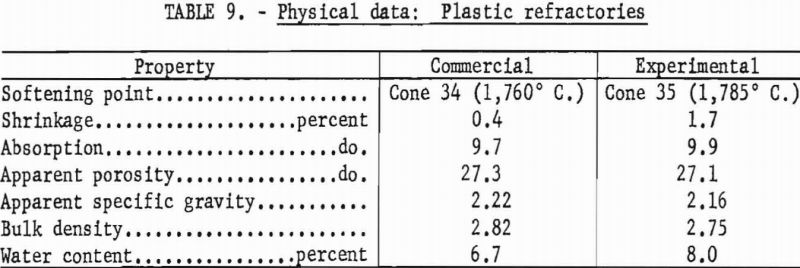
Enough water was added to permit pressing 4-¾- by ¾- by ¾-inch bars in hardened steel dies at 1,000 p.s.i. The bars were air-dried 24 hours, dried at 110° C. overnight, then fired to 1,600° C. in 5 hours with a 5-hour soak. Physical properties of the experimental plastic and a commercial plastic are given in table 9.
Both of the plastic refractories tested meet requirements for superduty service (spalling loss and slumping could not be determined). Their pyrometric cone equivalent was in excess of cone 32-½ (1,725° C.), the water content was less than 15 percent, and firing shrinkage was not more than 2.5 percent at 1,600° C.
Conclusions
Over a wide range of chemical and mineralogic compositions, the Alabama bauxitic kaolins studied in this investigation were suitable for high-duty refractory service, and by judicious blending most of them could be used in producing superduty refractories.
The greatest potential value of these kaolins lies in their utilization as components of refractory specialty products, such as mortars, plastics, and castables. However, the possibility of their use as raw materials in manufacturing ceramic products other than refractories should not be overlooked.
