Table of Contents
- Technique:
- Feed:
- Table Flotation Development:
- Skin Flotation:
- Agglomeration:
- Conditioning:
- Dilution:
- Capacity:
- APPLICATION:
- Broken Hill Concentrates:
- Test Work:
- Reagents:
- Lead Losses:
- Other Metals:
- Test Work Procedure
- Conditioning Device
- Extra Aeration:
- Wash Water and Slope of Table
- Technique of Tests
- Determination of the Most Suitable Reagents
- Effect of Air Jets
- Effect of Varying Conditioning Time
- Results of Final Tests
- Conclusion:
This paper deals with the application of table flotation to two unusual mineral dressing problems. Both problems involved the removal of interfering minerals at sizes too coarse for normal froth flotation.
The first problem was the removal of arsenical minerals from Broken Hill gravity concentrates without further size reduction. Table flotation without grinding of the original concentrates gave almost complete rejection of the arsenic from the concentrates. A typical test result was a reduction of arsenic in jig concentrate from 0.75% arsenic to 0.007% arsenic.
The second problem was to develop a method to produce a high grade calcium carbonate concentrate from coastal dune sands containing about 10% silica and about 80% calcium carbonate. The sands were about 30 mesh British Standard Screen (B.S.S.), and for economic and other reasons it was stipulated that the separation be made without grinding. Again, table flotation gave excellent results. A concentrate was produced containing 99.62% acid soluble material, representing a recovery of 98.69% of the calcium carbonate in the feed with a rejection of 96.44% of the silica.
The investigations were carried out at the Kalgoorlie Metallurgical Laboratory, under the Department of Mines filmed with oil. Air bubbles caused by the agitation are attracted and become attached to these oiled particles, which, with the attendant air bubbles, are attracted to each other and form agglomerates. The agglomerates although usually heavier than water are much lighter than the mineral, and when they are launched on to the surface of a shaking table float over the riffles.
Table flotation, therefore, consists of suitably conditioning the deslimed feed with reagents and fuel oil, then feeding the conditioned pulp on to a shaking table. The oiled particles, either in the form of agglomerates or by skin flotation, raft over the riffles to what is normally the slime side of the table. The non-oiled or wetted, particles remain submerged and travel along the riffles to what is normally the concentrate end of the table. The separation is particularly clean-cut and no middling is produced from the operation.
Technique:
To achieve the combined effects of skin flotation and rafting agglomerates it is necessary to have the right conditions. The requirements for successful table flotation are:
- The material must be relatively coarse and deslimed,
- The collector coating should be applied in a thick pulp with provision for thorough aeration,
- The conditioned pulp is diluted under conditions that will add more air,
- The capacity of tabling is highest if the majority of the material is floated.
- These requirements are discussed in more detail in the following paragraphs:
Feed:
The feed must be coarse, as fine material, or slime, does not skin float or form agglomerates to any extent. Slime must be removed from the feed because the rafting material travels over the table in much the same position of Western Australia and the C.S.I.R.O., at the School of Mines, Kalgoorlie. The investigations form the subjects of Reports Nos. 412 and 569
Before giving the details of the test work a description of the development and technique of table flotation will be given, as this operation is not widely known in Australia.
Table Flotation Development:
Table flotation is a combination of two mineral dressing operations—tabling and flotation. The selective oiling of mineral surfaces and the subsequent launching of the oiled particles at an air-water interface has been known from the earliest of times. For example, the principle of launching oiled pyrite on a water surface has long been used by Bolivian tin miners to reduce the sulphur content of their tin concentrates.
The significant point regarding this “skin” flotation is the relatively large size of particle that can be floated off in this manner. It is well known that froth flotation requires relatively fine grinding of the mineral grains, quite apart from considerations of liberation of valuable mineral from gangue. Attention in the last decade or so has, therefore, been turned to the development of a process whereby coarse particles of different minerals may be separated by the use of flotation principles. Two phenomena are used in table flotation:
Skin Flotation:
Oiled particles tend to skin float and, furthermore, in passing over and along the riffled deck of a shaking table submerged oiled particles break through the surface of the water and are carried off with the cross water. This skin float effect can be increased by the use of air or water jets impinging on to the table deck.
Agglomeration:
When an ore pulp is agitated with suitable reagents and an oil such as fuel oil, some of the particles become as the slime product in normal tabling, and any slime present in the feed would contaminate the floating product.
Conditioning:
Conditioning in a thick pulp is desirable for economy of reagents as well as the greater tendency to form agglomerates in a thick pulp.
The most suitable type of conditioning device for sandy deslimed pulp is a rotating drum with either an internal helix or lifter bars.
Dilution:
In the test work described later in this paper the pulp was diluted and allowed to fall about 6 in. into the feed box of the table. With sulphide flotation this gave sufficient aeration, but in the calcium, carbonate flotation work auxiliary air jets were necessary to get maximum recovery of oiled particles.
Capacity:
The capacity of the shaking table in table flotation is more dependent upon the amount of material moving along the riffles than upon the amount of material floating. The ideal condition would, therefore, be that 70 to 90% of the material is to be floated. In such a case the floating material travels rapidly in a broad almost unbroken sheet across the table. The wetted material travels along the riffles and, as this movement is slow, the capacity depends on the amount of material to be moved along the riffles.
APPLICATION:
For various reasons it is often convenient to separate minerals of different specific gravity at coarse sizes by gravity concentration. The concentrate of valuable mineral is, however, often contaminated by other minerals of like specific gravity. Also, minerals with specific gravities too close for gravity concentration may have to be separated at coarse sizes. Some minerals of low economic value cannot be concentrated by froth flotation due to the cost of fine grinding. In such cases table flotation has application, as this process can be used to separate minerals at relatively coarse sizes provided they are freed and can be selectively filmed with oil. In general it may be stated that the minerals which can be concentrated by froth flotation can be recovered in the relatively coarse state by table flotation.
The reagents used to condition the pulp in table flotation prior to selective filming with oil are much the same as used in froth flotation.
The upper limit of size of mineral grain that can be skin floated has not been determined, but galena 2 mm. in diameter was successfully rafted in the work on the Broken Hill concentrates.
Broken Hill Concentrates:
A request was received from Broken Hill Associated Smelters Proprietary Limited to investigate the removal of arsenopyrite in lead gravity concentrates from the Broken Hill mines. The method to be used was to avoid prior grinding of the concentrate.
Test Work:
Four samples were received, consisting of jig and table concentrates from the South and North mines. The concentrates as received were ideally suited to table flotation for the following reasons:
- The arsenical minerals were substantially freed from the galena.
- The concentrates were coarse and contained no slime.
- Galena was, naturally, the predominant mineral. This mineral is relatively easy to float, and arsenopyrite is relatively easy to depress, so the greater bulk of the material was floated.
Little information was available regarding the differential table flotation of sulphide minerals and a considerable amount of preliminary work was necessary to establish a technique.
A rotating drum-type conditioner was constructed by joining two bell jars with a rubber sleeve and placing a metal helix in the open ended cylinder so formed. The device was rotated in a tilting frame using the necks of the bell jars as trunnions. After the required time of conditioning the frame was tilted, diluting water was added through the raised neck, and the material was discharged at a relatively steady rate with the conditioner rotating.
The pulp from the conditioner was allowed to fall about 6 in. into the feed box of the table. The amount of cross water was not critical but too much caused excessive riffling action and some floating galena dropped but of contact with the air. Some riffling action was necessary to drop out lightly held locked particles and mechanically entrained non-lead minerals. This riffling action also allowed submerged galena particles to be presented to the air-water interfaces.
The fine galena formed air-mineral agglomerates, but the coarse galena rafted over the table as individual particles. A definite split was obtained; the galena moved across the deck in an almost unbroken sheet, and the non rafting gangue minerals, zinc, iron, and arsenical, minerals travelled along the riffles below the surface of the water. The wash water falling onto the table deck just above the topmost riffle cleat had the effect of exposing to air any entrapped galena in the tailing moving along this riffle.
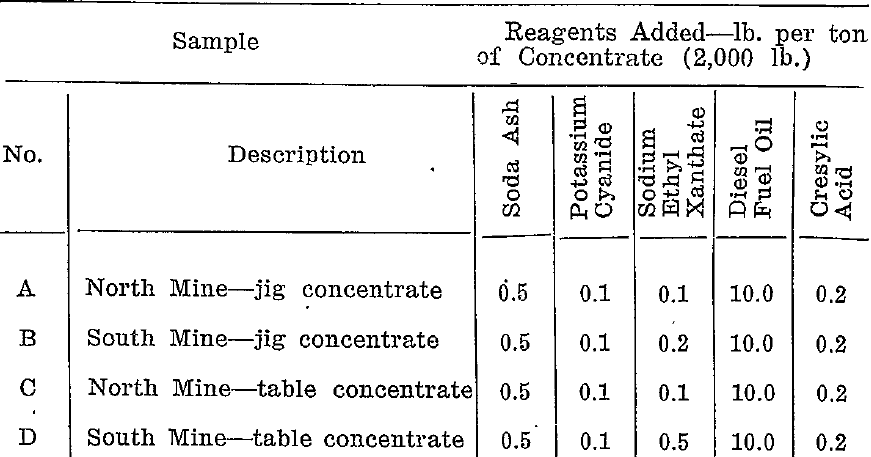
A series of tests was carried out on each of the four samples to determine the reagent conditions necessary to depress the arsenopyrite and allow the galena to float. Table 1 gives the most suitable reagent combinations.
Preliminary tests had established that soda ash, cyanide, fuel, oil and cresylic acid in the quantities shown gave satisfactory results. Control was effected by varying the amount of ethyl xanthate.
Table 1. Reagent Quantities for Table Flotation of Lead Concentrate
Notes:
- The soda ash and potassium cyanide were added together.
- The fuel oil and cresylic acid were added as a mixture.
- The reagents were added in the order from left to right allowing 10 minutes conditioning between additions.
Inspection of the tailings from early tests showed that considerable amounts of locked galena grains, and galena with “old” surfaces, were present. In subsequent tests the procedure adopted was to condition 1,000 gm. of the sample, and table float, producing a concentrate and a tailing. The tailing was then crushed to 20 or 40 mesh as indicated in the table of test results (Table 2). The crushed tailing was then conditioned with the same quantities of reagents as before and table floated. The concentrate from this retreatment of the original tailing was termed middling. The small quantity of tailing made difficult the accurate
Table 2 – Results of Table Flotation of Lead Concentrates.

control of reagent quantities in the retreatment and better results would almost certainly be obtained with tests on a larger scale.
Table 2 gives the results obtained using the reagents and conditions given in Table I.
NOTES ON THE TEST WORK
Reagents:
Ethyl Xanthate: It was necessary to vary the amount of ethyl xanthate added to the various samples to get satisfactory results.
Depressant: No attempt was made to vary the amount of cyanide added. The pH of the conditioned pulp was between 8 and 9.
Oil: Diesel fuel oil was found quite effective, especially if a little cresylic acid was added. The quantities of 10 lb. of fuel oil and 0.2 lb. of cresylic acid were arbitrary. Due to lack of sample it was not possible to carry out a series of tests to determine the minimum of oil requirement.
Lead Losses:
In Tailings: The lead losses in the tailing varied from 0.12 to 0.47%. Most of the sulphide lead in the tailing was in the form of locked particles, that is, small grains of galena attached to other sulphides or gangue minerals. Finer crushing and froth flotation would enable recovery of most of this lead. The oxy-salts of lead did not float under the conditions of the tests, but observation of the tailing product on the table showed that it would be possible to take off a heavy mineral fraction from this tailing. The wetted material graded into normal bands and any non-rafting galena and the oxy-salts of lead could be taken off as in normal tabling.
In Middling: The concentrate (middling) from the cleaning of the initial tailing after crushing, contained considerable arsenic and finer grinding is indicated to free the locked galena, for recovery by table flotation or by froth flotation.
Other Metals:
Arsenic: This metal was reduced to between 0.03% and 0.004% in all four samples without prior grinding.
Antimony and Silver: These metals followed the lead.
Zinc: Approximately half the zinc was removed from the material.
Gangue: The gangue minerals entered the tailing almost completely.
DUNE SANDS
The laboratory had been requested by the Department of Industrial Development, Perth, to develop a method for the production of a high grade calcium carbonate concentrate from dune sands at Mullalloo Beach, north of Perth. It was stipulated that the separation be made without first grinding the material as the concentrate was to be used to make lime, and dust losses would be severe on a fine product.
General Information
The Mullalloo beach sand sample contained 81.4% calcium carbonate in the form of shell fragments, and 9.46% silica. The silica was mainly in the form of sponge spicules with some quartz grains. The shell fragments contained about 6% magnesium carbonate. Shell-forming organisms precipitate varying amounts of magnesium carbonate with the calcium carbonate during the formation of calcareous skeletons. Therefore it would not be possible to make a mechanical separation of the two carbonates. Examination of products separated under the microscope showed that the siliceous material in the sand was free from the carbonates at the grain size of the sand.
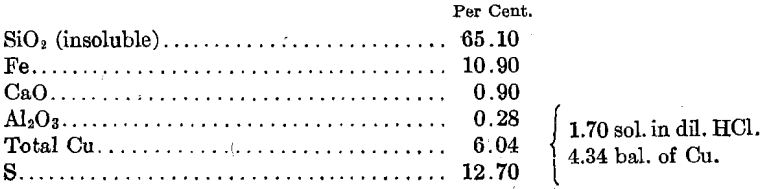
A chemical analysis of the sample is given in Table 3 and a screen analysis in Table 4.
The dune sand admirably fulfilled the requirement for table flotation. The material was relatively coarse and free from slime, the silica and carbonate were free, and calcium carbonate is easier to float than silica.
Test Work Procedure
The programme of test work on the dune sands was as follows:
- Tests to establish a suitable technique.
- Determination of the most suitable reagents.
- Determination of the minimum quantities of reagents for best recovery and grade.
- Effect of air jets.
- Effect of varying conditioning time.
- More details about the test work are given in the following paragraphs.
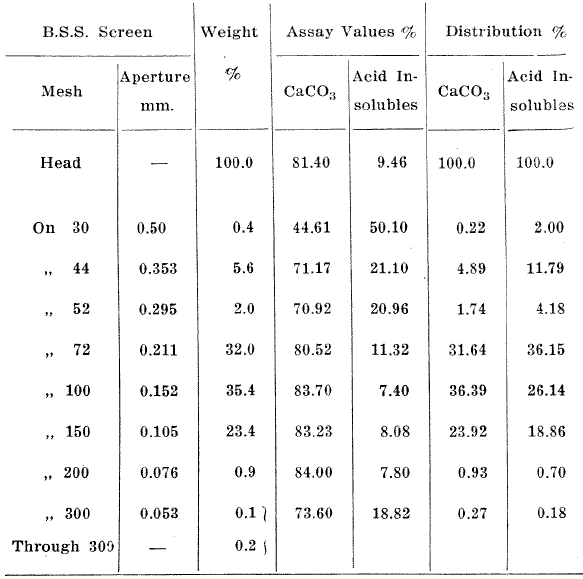
Table 4. Screen Analysis of Dune Sands
Conditioning Device
Several types were tried but the most suitable was a rotating drum with longitudinal lifter bars. A chute moved by means of a rack and pinion allowed the material to be fed from the rotating drum to the table. The drum and lifter bars were made from 1/8in. perspex and the unit was mounted on rollers. (Fig. 1).
The optimum speed of rotation with 1,000 gr. of sand at 60.5% solids was 14 revolutions per minute.
Extra Aeration:
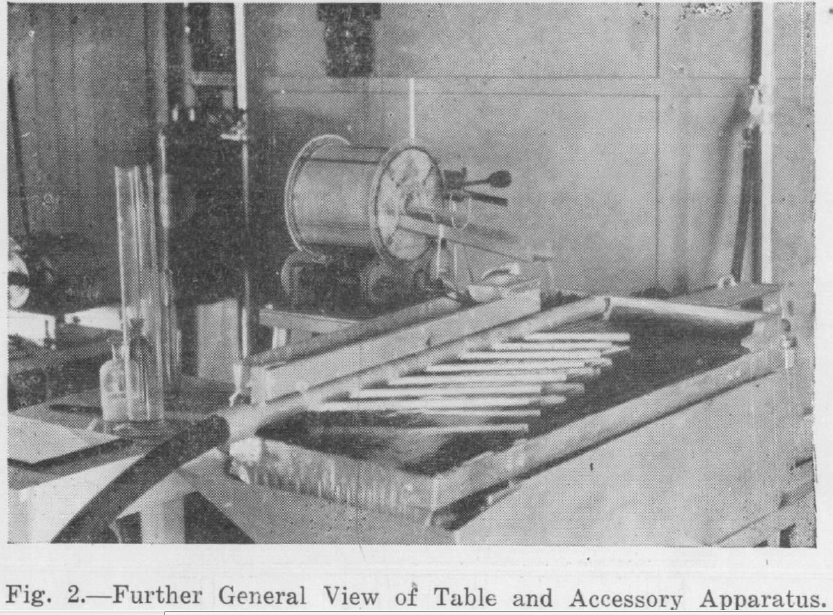
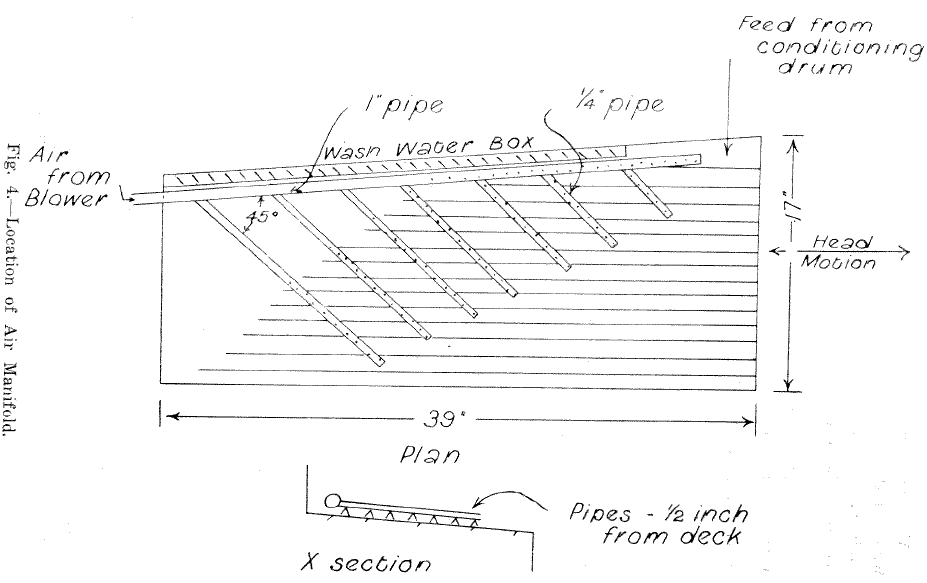
Preliminary tests using the drum conditioning device and attempting the table flotation on the unmodified deck of a laboratory Wilfley table were not particularly successful. A considerable portion of the carbonates, although oiled, did not raft. Experiments with a hand held jet of compressed air showed that air jets would assist the flotation. Allan described the use of a system of air jets on the decks of shaking tables used for table flotation. Accordingly a manifold was constructed and attached to the table as shown in Fig. 2. The manifold had 220 holes 1/16th in. dia., ¼ in. apart, and was supplied with compressed air from a cycloidal type blower through a 1 in. dia. hose at 2 in. of mercury pressure.
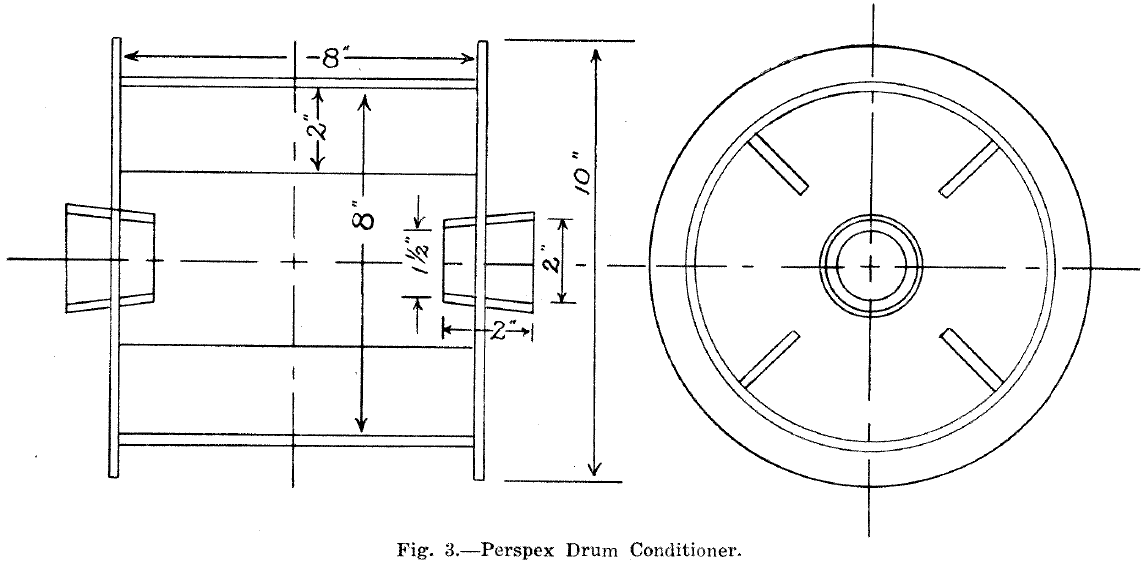
Table Flotation of Arsenopyrite and Silica
Wash Water and Slope of Table
Although the amount of cross water was not critical it was found best to keep the flow rate such that the rafting carbonates flowed over the riffles in an unbroken film without “boiling” in the riffles spaces.
The slope of the table was kept at 0.5 in. per ft. This was not critical as the separation was very sharp.
Technique of Tests
Pulp Ratio: The pulp ratio during conditioning was 60.5% solids. Pulp thicker than this did not cascade freely.
Addition of Reagents: After placing the required amount of sand and water in the drum the reagents were added in the sequence noted in Table 5.
Flotation: After conditioning for the required time the pulp was fed from the drum by means of an adjustable chute. Diluting water was added, and the diluted pulp was allowed to fall about 4 in. on to the deck of the table.
The feed box was removed as it was found that greater aeration occurred with this removed. More than half the carbonates rafted and moved over to where the slime is normally removed. The remainder of the carbonates plus the silica moved along the topmost riffle cleats where the air jets impinging on the water surface gave the oiled carbonates the opportunity to raft. The wetted particles moved along the riffles and the separation in almost every test was clean and no middling was made.
Throughout the table flotation tests on this material the carbonates did not form any large proportion of agglomerates even when conditioned for several hours. Most of the carbonates rafted over as individually floating grains.
Determination of the Most Suitable Reagents
A series of tests was carried out using combinations of soda ash, sodium meta-silicate, oleic acid, sulphonated castor oil, eucalyptus oil, and fuel oil. The most satisfactory combination was sulphonated castor oil and diesel fuel oil.
Determination of Minimum Quantities of Reagents:
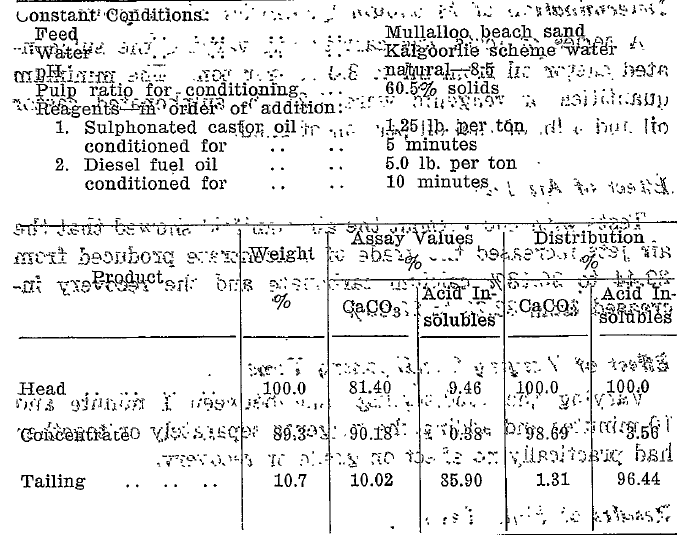
A series of tests was carried out varying the sulphonated castor oil from nil to 3.0 lb. per ton. The minimum quantities of reagents were 1.25, lb. sulphonated castor oil and 5 lb. of fuel oil per ton of sand.
Effect of Air Jets
Tests with and without the air manifold showed that the air jets increased the grade of concentrate produced from 89.44 to 90.18% calcium carbonate and the recovery increased from 88.28 to 98.69%.
Effect of Varying Conditioning Time
Varying the conditioning time between 1 minute and 10 minutes and adding the reagents separately or together had practically no effect on grade or recovery.
Results of Final Tests
Table 5 gives the conditions of, the final tests and the results obtained.
The test result (Table 5) shows that table flotation can be applied to this material in the unground state to yield a high grade calcium carbonate concentrate low in silica.
The calculated maximum grade of calcium carbonate obtained from the sand is 92.23%. The grade of 90.18% calcium carbonate is, therefore, 97.76, % of the possible.
Reagent consumption is low for this type of mineral beneficiation, and the reagents are cheap and easy to obtain.
The rafting concentrate is easily collected and there is no difficult froth to break down.
Table 5- Reagents and Conditions for Table Flotation of Dune Sands
Conclusion:
The possibilities of this method of mineral beneficiation have been demonstrated in the two investigations discussed. This method of removal of minerals from relatively course material should have a wide field of application. The disadvantages of the method are that the material must, be coarse and deslimed, and the products are contaminated, with diesel fuel oil.
Acknowledgements:
The author wishes to thank the Director of the Western Australian School of Mines for permission to publish this paper and for assistance in its preparation. Thanks are also due to the Director, Department of Industrial Development of Industrial Development, Perth, and the General Manager, Broken Hill Associated Smelters for permission to use data from reports of investigation carried out at their request. The author also acknowledged the assistance of the staff of the Kalgoorlie Metallurgical Laboratory in particular E. E. Hughes and A. F. Griffin, who took an active part in the experimental work.
THE APPLICATION OF TABLE FLOTATION TO THE REMOVAL OF ARSENOPYRITE FROM GALENA CONCENTRATES AND SILICA FROM LIME SANDS, BY C. H. S. MEHMtRY
