Table of Contents
- Materials
- Experimental Procedure and Results
- Small-Scale Tests
- Initial Coextraction
- Separation of Tantalum from Columbium
- Procedure for High-Columbium Materials
- Procedure for High-Tantalum Materials
- Assay Procedure for Equal Amounts of Tantalum and Columbium
- Separation of Tantalum from Columbium in Special Cases
- Reuse of Solutions
- Recycling Feed Solutions
- Reconditioning Methyl Isobutyl Ketone
- Rate of Transfer Tests
- Large-Scale Tests
- Apparatus
- Procedure and Results for Mixer-Settler Column
- Stepwise Tests, Mixer-Settler Column with Gravity Feed System
- Stepwise Tests, Mixer-Settler Column With Pump Feed system
- Continuous Runs, Mixer-Settler Columns in Cascade
- Procedure and Results of Pump-Mix Mixer-Settler Tests
- Stepwise Tests with Single- and Two-Stage Units
- Continuous Test, Pump-Mix Mixer-Settler Units in Array
- Continuous Test, Recycling Aqueous Feed Solution
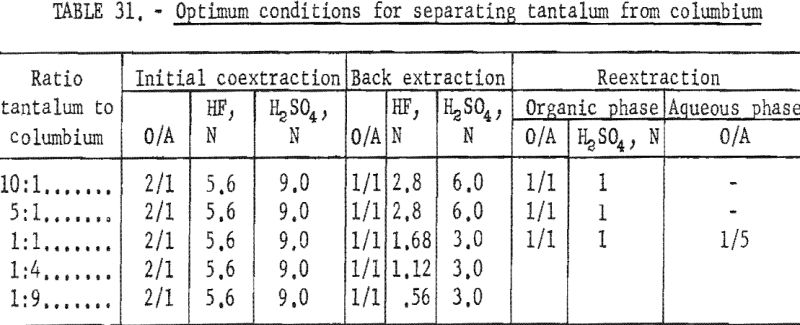
Results of this investigation demonstrate that high-purity columbium and tantalum compounds can be obtained by application of the solvent extraction separation system: Hydrofluoric acid-sulfuric acid-methyl isobutyl ketone. This system is unique in that it not only separates columbium from tantalum, but separates these two metals from other metallic impurities. The system is effective for separating the metals irrespective of the columbium-to-tantalum ratio of the starting material. The conditions chosen to be the best by the authors are tabulated in table 31.
These investigations show that this system is very effective for separating tantalum and columbium when used in large-scale continuous equipment. The mixer-settler units described are of simple design and are inexpensive to fabricate. The pump-mix mixer-settler is more efficient than the mixer-settler column. Either unit will produce high-purity columbium and tantalum compounds continuously.
The cost of the separating may be reduced by recycling the spent feed from the initial coextraction and the organic phase from the reextraction of the aqueous phase. The sulfuric acid solution from the reextraction of the organic phase is too dilute to be of any value. The solvent may be reconditioned and reused without impairing the efficiency of the separation or the purity of the products.
Procedures for separating columbium and tantalum have been developed by the Bureau of Mines as part of a broad research program on the utilization of low-grade ores of columbium and tantalum. Columbium and tantalum compounds of greater than 99 percent purity have been obtained by application of the solvent extraction separation system: Hydrofluoric acid-sulfuric acid-methyl isobutyl ketone. This system is unique in that it not only separates columbium from tantalum, but also separates these two metals from other metallic impurities. The system is effective for separating the metals irrespective of the tantalum and columbium ratio of the starting materials. Investigations in which the spent feed obtained from the initial coextraction step was recycled to the system indicated that efficiency was increased, and cost was reduced without impairing product purity. Continuous tests demonstrated that this solvent extraction system could be used in large-scale equipment without loss in efficiency or in purity of products.
The separation of columbium and tantalum has been difficult because of the similarity in their chemical behavior. Many methods have been proposed for separating these two elements, but most methods either did not achieve satisfactory separation or were tedious and time consuming. The Marignac process developed in 1866 was the only method used commercially until recently. This separation procedure is based on the difference in solubility in aqueous solution of potassium tantalum fluoride and potassium columbium oxyfluoride. Current commercial practice appears to favor solvent extraction as a method for separating tantalum from columbium. Several efficient methods for separating columbium from tantalum by solvent extraction were reported earlier from this laboratory.
Investigations have been continued in order to develop a process that would not only effect a separation of columbium from tantalum, but would also separate the two metals from other metallic impurities normally extracted with them from ores and concentrates. The hydrofluoric acid-sulfuric acid-methyl isobutyl ketone solvent extraction system fulfills these requirements. This report describes the investigations made to determine the optimum conditions for achieving separation. Results of both shaker-scale and continuous countercurrent tests are given.
Materials
The columbium-tantalum mixtures used in this investigation were hydrated oxides obtained by the hydrolysis of anhydrous tantalum and columbium chlorides. Drying these hydrated oxides at 150° C, yielded a product that contained approximately 15 percent water. Two types of material were used in the small-scale studies: (1) Synthetic mixtures of metal oxides prepared by the hydrolysis of anhydrous chlorides obtained by chlorination of individual pure metals, and (2) mixtures in naturally occurring proportions resulting from the hydrolysis of chlorides obtained by the chlorination of various ores. Synthetic mixtures were prepared because natural materials containing high tantalum- to-columbium ratios were not available. Only natural mixtures were used in the large-scale tests.
Both reagent and technical grade hydrofluoric acid, technical grade sulfuric acid, and methyl isobutyl ketone were used as received.
Experimental Procedure and Results
The solvent extraction system for separating tantalum and columbium that was investigated is applicable to all mixtures of tantalum and columbium regardless of the ratio in the starting material. This process is based on the principle of coextraction of both tantalum and columbium from the aqueous feed solution into the organic phase to separate them from impurities, and on the principle of preferential back extraction of the metal values from the organic phase with dilute acids to separate columbium from tantalum. The investigative work, both small- and large-scale, was based on this plan.
The aqueous feed solutions for the experimental investigations were prepared by dissolving tantalum-columbium hydrated oxides in hydrofluoric-sulfuric acid mixtures. Separation of the two metals was achieved in four steps. First; the aqueous feed solution was contacted with two volumes of methyl isobutyl ketone, which coextracted the columbium and tantalum fluoride complexes, leaving the metallic impurities in the aqueous phase. Second, the metal-laden solvent was contacted with a dilute hydrofluoric acid-sulfuric acid solution; this back extraction effected partial separation of the metal values. The organic phase from this operation contained tantalum compounds with about 5 percent columbium, and the aqueous phase contained columbium compounds with about 5 percent tantalum. In the third step, the organic phase was reextracted with dilute sulfuric acid to purify the tantalum by stripping out the columbium. In the fourth step, the aqueous solution from the back extraction was reextracted with a small amount of fresh organic solvent to
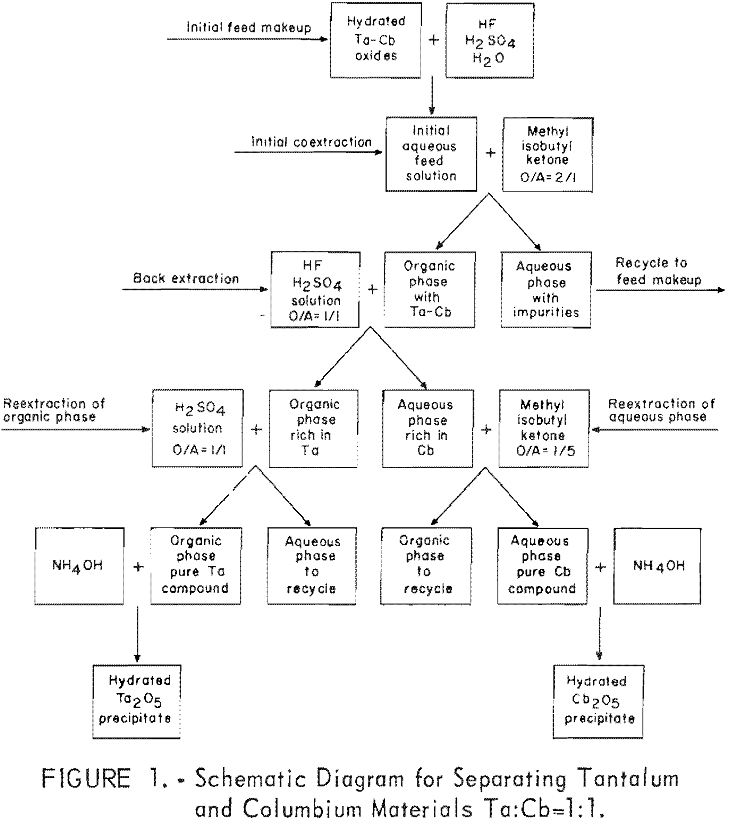
yield pure columbium compounds. The procedure used for this work is outlined by the schematic diagram, figure 1.
Small-Scale Tests
Small-scale tests were conducted to establish the best operating conditions. These conditions then were used for large-scale separation tests run in two different devices for continuous contact, mixer-settler columns and pump-mix mixer-settlers.
Small-scale tests were conducted on starting materials containing tantalum-to-columbium ratios ranging from 1:9 to 10:1. The operational variables investigated for this system were conditions necessary for initial coextraction of the metals, and for the separation of tantalum from columbium by preferential back extraction.
Initial Coextraction
The variables investigated for the initial coextraction were metal concentration of the aqueous feed solution, acid concentration of the aqueous solution, organic-to-aqueous ratio, and effectiveness of initial coextraction in removing metallic impurities.
In the procedure for initial coextraction tests, aqueous feed solutions were prepared by dissolving hydrated oxides of tantalum and columbium in concentrated hydrofluoric acid. Calculated quantities of sulfuric acid and water were added to obtain the desired acid and metal concentrations in the feed solution. Two volumes of methyl isobutyl ketone were shaken with one volume of aqueous solution in 500-milliliter polyethylene bottles for 15 minutes on a wrist-action shaker. The mixture was then transferred to a separatory funnel, and the organic and aqueous phases were drawn off into appropriate containers. Ammonium hydroxide was added to each phase, and the metal hydroxides were collected on a filter and ignited at 800° C. The oxides were weighed. The values reported as percent extracted were calculated as follows:
weight organic phase precipitate/weight starting oxides x 100
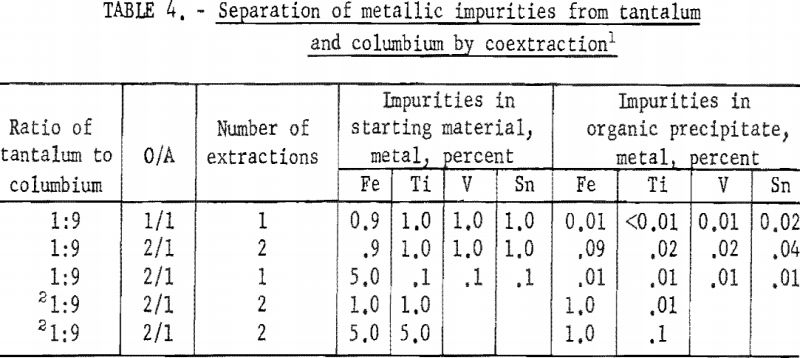
Tests were run to determine how much metal could be coextracted from the aqueous feed solution into the organic phase. Table 1 shows the results of these tests. Tantalum and columbium were transferred quantitatively from the aqueous feed solution to the organic phase when the oxide concentration in the aqueous feed solution was 100 grams per liter or less. Therefore, the concentration in aqueous feed solutions was maintained at no more than this figure for all subsequent tests with the assumption that satisfactory transfer would result.
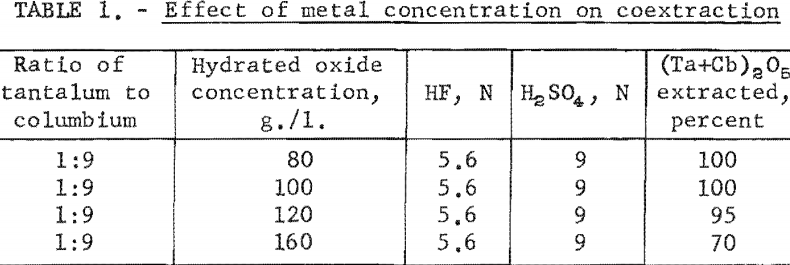
The effect of varying the concentration of hydrofluoric and sulfuric acids in the initial aqueous feed solution was investigated, and the results appear in table 2.
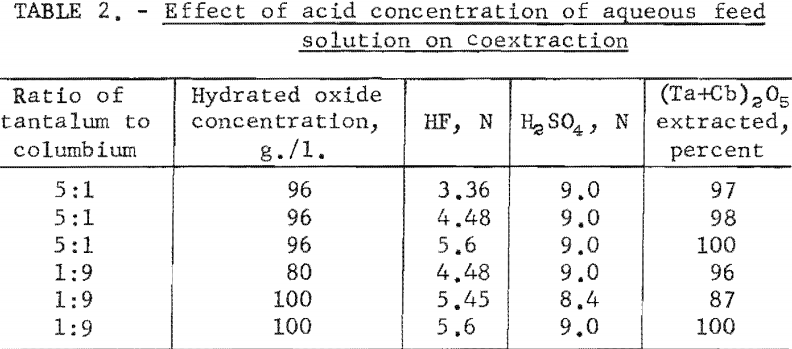
The effect of varying the organic-to-aqueous ratio was determined. The results of this variation are shown in table 3.
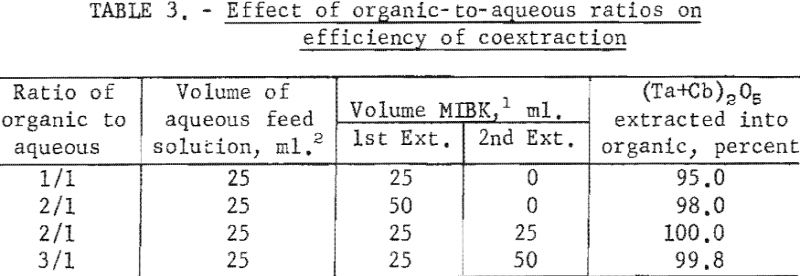
Tests were run to determine the effectiveness of the initial coextraction procedure for removing metallic impurities from tantalum and columbium. When tantalum and columbium are extracted from ores by chlorination processes, small amounts of iron and titanium are likely to be present in the mixed chlorides as impurities. For these tests, feed solutions were prepared from a natural mixture of hydrated tantalum and columbium oxides. In two tests, iron and titanium were added to the natural mixture in amounts of 1 and 5 percent each. The prepared feed solutions were carried through the initial coextraction procedure under the conditions determined to be best in previous tests. Spectrographic analyses of the starting material and of the metals precipitated from the organic phase following extraction are shown in table 4.
Experimental data indicate that 100 grams per liter was the maximum concentration that could be extracted completely from the aqueous feed solution into the organic phase under the conditions used for these tests. The minimum acid concentration necessary to transfer quantitatively both tantalum and columbium, regardless of their ratio in the starting material, was 5.6 N hydrofluoric acid and 9 N sulfuric acid. The data from table 3 show that two extractions with equal volumes of methyl isobutyl ketone (MIBK) is more efficient than one extraction with two volumes of methyl isobutyl ketone. The tests reported in table 4 indicate that if iron is present in the initial aqueous feed solution, it would be transferred into the organic phase during the second extraction with an equal volume of methyl isobutyl ketone. It would be preferable to sacrifice a small amount of tantalum and columbium for the sake of purity, and contact the aqueous feed solution once with two volumes of methyl isobutyl ketone.
Separation of Tantalum from Columbium
The determining factor in selecting the acid concentration of the back extracting solutions and the number of back extractions necessary to effect separation was the ratio of tantalum to columbium in the starting material. The variables investigated for the actual separation of tantalum from columbium by preferential back extraction for each ratio of tantalum to columbium were acid concentrations of the aqueous extracting solutions, organic-to-aqueous ratios, and number of extractions necessary for obtaining pure tantalum and/or columbium compounds.
The data reported for this work do riot include data for the initial coextraction. The conditions for initial coextraction for each test were as follows: The aqueous feed solution contained 100 grams per liter of hydrated oxides; the hydrofluoric and sulfuric acid concentrations were 5.6 and 9 N, respectively; the organic-to-aqueous ratio was 2 volumes to 1; one extraction was made.
The values reported for the percent recovery and percent recycle in the tables of separation test results were calculated as follows:
Recovery, percent = wt. recovered in phase (O or A) x (pct. purity, Ta2O5 or Cb2O5)/(wt. total sample) x (pct. Ta2O5 or pct. Cb2O5) x 100
Recycle, percent = weight recovered in extracting phase/weight total sample x 100
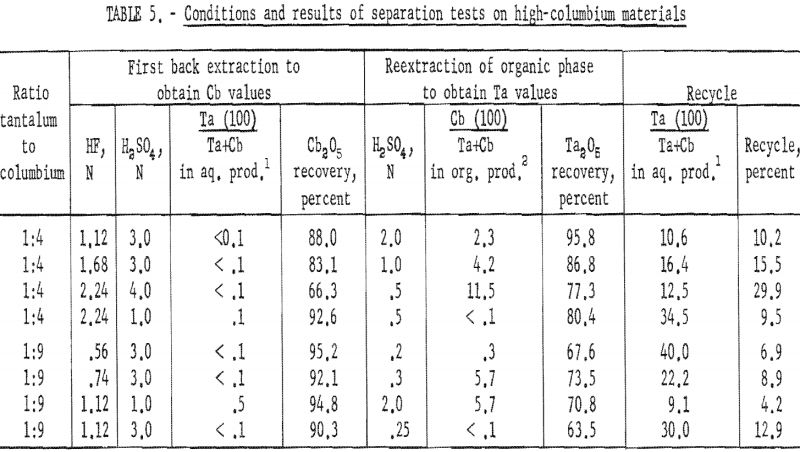
Procedure for High-Columbium Materials
Natural mixtures of tantalum and columbium in ratios of 1:4 and 1:9 were used to investigate procedures for high-columbium materials. An equal volume of acid solution of known hydrofluoric acid and sulfuric acid concentration was added to the organic phase from the initial coextraction, and the two phases were shaken for 15 minutes and then separated. The tantalum and columbium present in each phase were precipitated with ammonium hydroxide. These hydroxides were collected on a filter and ignited at 800° C. The ignited oxides were weighed and then analyzed for tantalum and columbium content by X-ray spectroscopy.
Results of these tests, shown in table 5, indicate that the optimum acid concentration of the back extraction solutions in respect to the purity and recovery of columbium compounds would be 1.12 N HF – 3 N H2SO4 for materials with Ta:Cb ratio of 1:4 and 0.56 N HF – 3 N H2SO4 for materials with Ta:Cb ratio of 1:9.
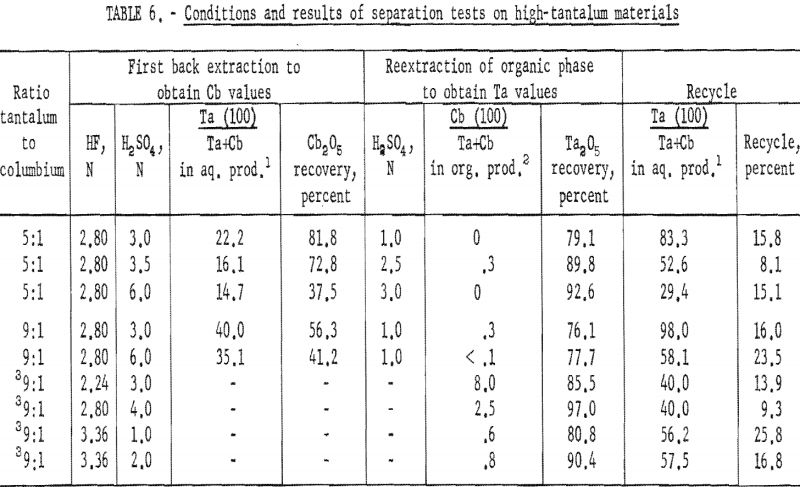
Procedure for High-Tantalum Materials
Synthetic mixtures in which the tantalum-to-columbium ratios were 5:1 and 10:1 were used to investigate procedures for high-tantalum materials. An equal volume of dilute hydrofluoric-sulfuric acid solution was added to the organic phase from initial coextraction; the mixture was shaken for 15 minutes, and the two phases were separated. The organic phase was then reextracted by shaking it for 15 minutes with an equal volume of dilute sulfuric acid. Following separation of the phases, the metals in the resultant two phases were precipitated with ammonium hydroxide, collected, and ignited at 800° C. The ignited oxides were weighed and then analyzed for tantalum and columbium content.
Conditions and results of these tests are given in table 6. Best conditions were selected on the basis of purity and recovery of tantalum product. One extraction with an aqueous solution of 3.36 N HF – 2 N H2SO4 resulted in tantalum products containing less than 1 percent columbium. Two extractions were necessary for recovering tantalum products containing less than 0.1 percent columbium. The optimum acid concentration for the first back extraction solution was 2.8 N HF – 6 N H2SO4; the optimum concentration for the reextraction solution was 1 N H2SO4.
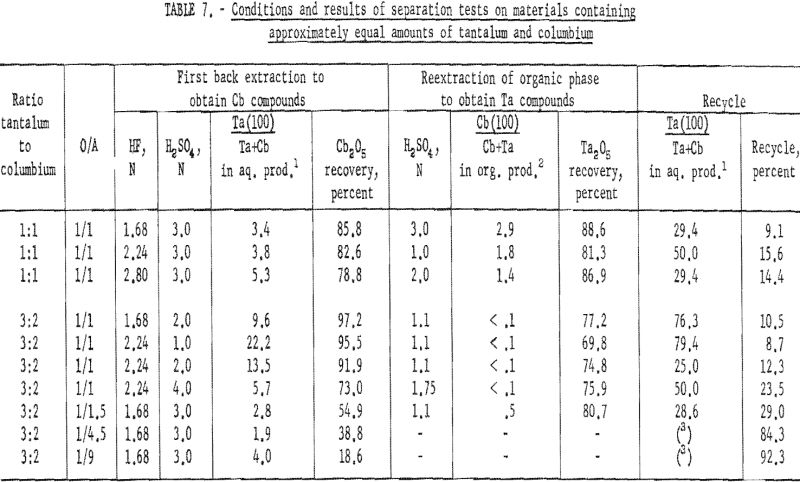
Assay Procedure for Equal Amounts of Tantalum and Columbium
A synthetic mixture of tantalum and columbium in the ratio of 1:1 and natural mixtures in which the two metals occur in 3:2 and 1:1 tantalum-to-columbium ratios were used for this investigation. The organic phase from initial coextraction was back extracted by shaking it with a dilute hydrofluoric-sulfuric acid solution for 15 minutes and separating the two phases. The resultant organic solution was reextracted by shaking with an equal volume of dilute sulfuric acid; following separation of the phases, ammonium hydroxide was added to each phase. The precipitated metal hydroxides were collected and ignited at 800° C. Table 7 shows the weights of oxides recovered and their tantalum and columbium content.
This procedure of preferential back extraction proved satisfactory for the recovery of a pure tantalum product. The best acid concentration in the back extraction solution was 1.68 N HF – 2 N H2SO4; the optimum sulfuric acid concentration in the reextraction solution was 1.1 N. Neither variation of acid concentrations nor of organic-to-aqueous ratios yielded a columbium product free of tantalum in one back extraction.
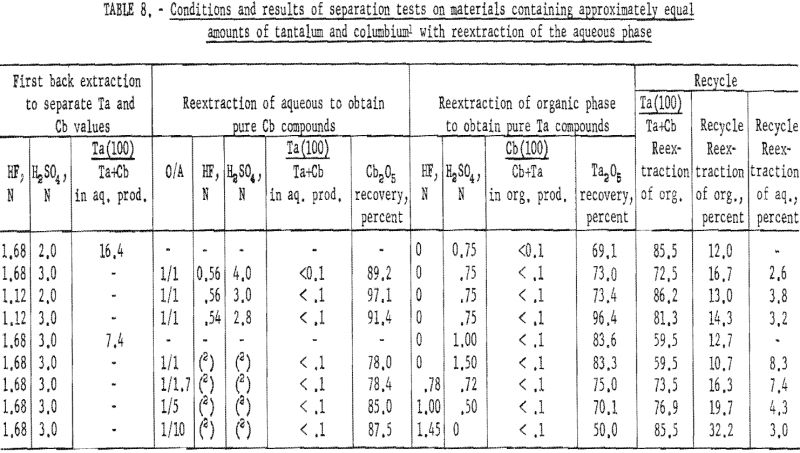
Two methods of reextracting the aqueous solution following the first back extraction were tested: (1) Varying acid concentrations in the aqueous phase, and (2) varying organic-to-aqueous ratios. In the first, the acid concentration of the aqueous solution was determined volumetrically. The total acid concentration was determined by titrating 1-milliliter aliquots of the solution with a standard base. The concentration of total fluoride was then determined by the method of Willard and Winter. The sulfuric acid concentration was approximated from the difference of the two values.
The aqueous solution was then diluted to a desired acid concentration and shaken with an equal volume of methyl isobutyl ketone for 15 minutes; the two phases were separated in the usual manner. The metals present in the resultant two phases were precipitated as hydroxides, collected, and ignited.
The second method required the addition of methyl isobutyl ketone to the undiluted aqueous solution from the first back extraction in various organic-to-aqueous ratios. The liquids were shaken, and the two phases separated. Ammonium hydroxide was added to the resultant organic and aqueous solutions, and the metal hydroxides were collected and ignited at 800° C.
Results of these tests are shown in table 8. The best back extraction conditions were 1.68 N HF – 3 N H2SO4. Satisfactory recovery of columbium resulted from reextraction of the aqueous solution from back extraction with fresh methyl isobutyl ketone in an organic-to-aqueous ratio of 1:5. Tantalum products containing less than 0.1 percent columbium were recovered by reextracting the organic solution from back extraction with an equal volume of 1 N H2SO4.
Separation of Tantalum from Columbium in Special Cases
After the system applicable to several starting materials had been established, variables were investigated that would make the system more efficient for special starting materials. These starting materials were either high in tantalum or high in columbium. The purpose of the tests was to recover the predominant metal in pure form and disregard recovery of the minor constituent. Separation of tantalum from columbium was effected by extraction of tantalum only from dilute hydrofluoric-sulfuric acid feed solutions with methyl isobutyl ketone. When this extraction principle was applied to high-tantalum materials, the tantalum that extracted into the solvent phase contained about 1 percent columbium. The organic phase was back extracted with dilute sulfuric acid to reduce columbium contamination to 0.1 percent. Separation of columbium from tantalum in high-columbium materials was effected in one extraction with solvent. The columbium remained in the feed solution and subsequently could be separated from metallic impurities by transferring it to fresh methyl isobutyl ketone. For this second step, the hydrofluoric acid concentration of the feed solution had to be increased to 5.6 N.
Starting materials for these tests were artificial and natural mixtures of hydrated oxides with tantalum-to-columbium ratios of 33:1, 4.5:1, and 1:9. Feed solutions were prepared by dissolving the hydrated oxides in concentrated hydrofluoric acid and diluting the solution with sulfuric acid and water to result in the desired acid and metal concentrations. The feed solution was contacted with two volumes of methyl isobutyl ketone by shaking for 15 minutes. The phases were allowed to separate and then were drawn off into appropriate containers. Ammonium hydroxide was added to each phase to precipitate the metals present. The hydroxides were collected on a filter and ignited at 800° C. The ignited oxides were weighed and analyzed for tantalum and columbium content.
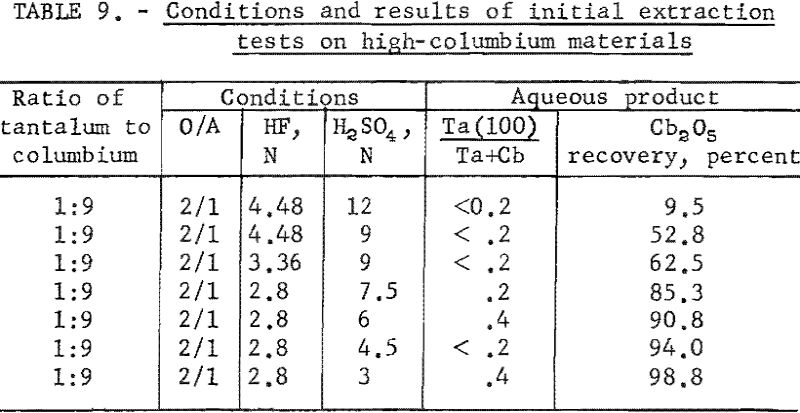
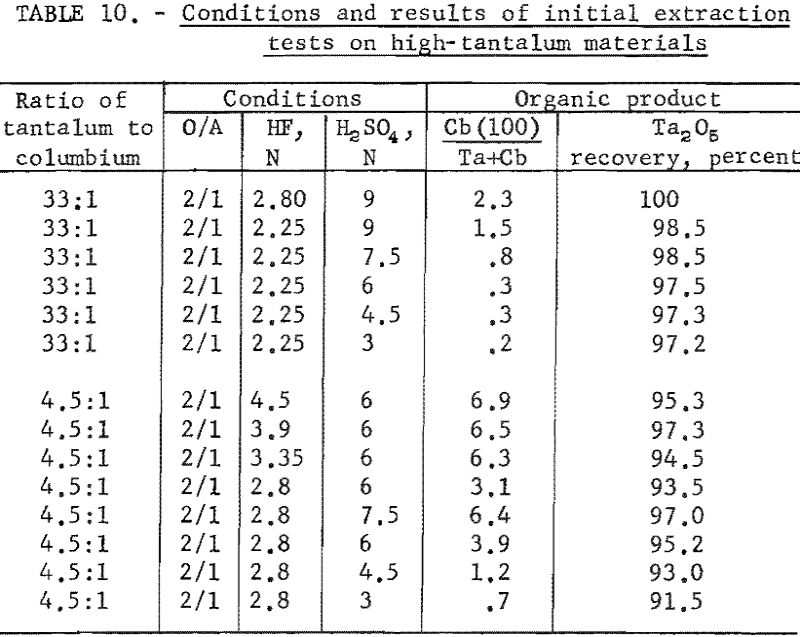
Variables investigated in the initial extraction tests were the concentrations of hydrofluoric acid and sulfuric acid in the feed solutions. Conditions and results of high-columbium tests are in table 9. A columbium product containing less than 0.2 percent tantalum was collected from high-columbium materials in a single extraction from 2.8 N HF – 4.5 N H2SO4 feed solutions. Results of high-tantalum tests in table 10 show that tantalum products collected from the organic solutions following initial extraction contained 0.2 to 2.0 percent columbium oxide when the tantalum-to-columbium ratio of the starting material was 33:1. When the feed solution contained the lower ratio of 4.5:1, the tantalum product contained 1 to 7 percent columbium oxide.
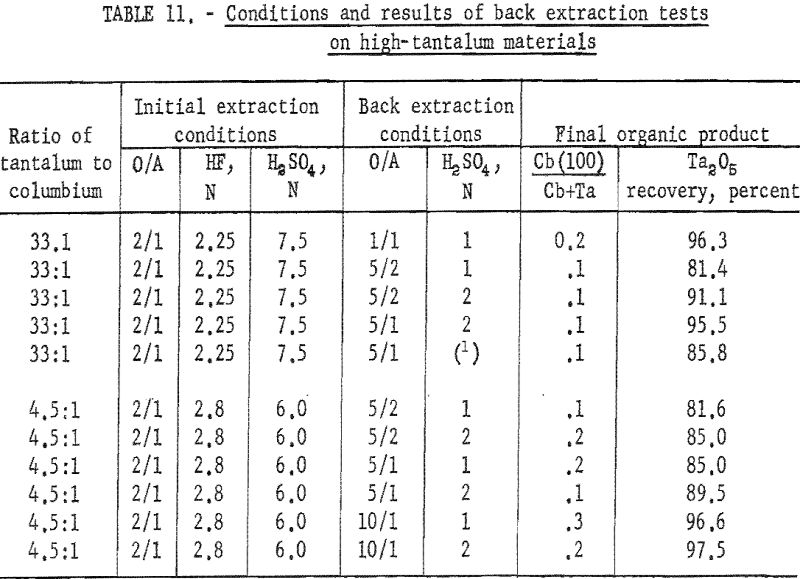
The organic phase resulting from initial extraction tests of high-tantalum materials was back extracted with dilute sulfuric acid to remove columbium impurity. Variables for these tests were acid concentration of the back extraction solution and organic-to-aqueous ratios. In the procedure tested, feed solutions were prepared and contacted with two volumes of solvent. The resulting organic solution was then shaken with the desired volume of aqueous back-extraction solution, and the two phases were allowed to separate. The metals present in the final organic phases were collected by ammonium hydroxide precipitation. Table 11 includes the conditions of initial extraction and the conditions and results of back extraction of the organic phase to purify tantalum products. Back extraction with 2 N H2SO4 at an organic-to-aqueous ratio of 5:1 results in products containing 0.1 percent columbium from starting materials of 33:1 tantalum-to-columbium ratio. Back extraction of the organic phase with 2 N H2SO4 at the organic-to-aqueous ratio of 10:1 results in satisfactory recovery of tantalum products from starting materials containing tantalum-to-columbium ratios of 4.5:1.
Satisfactory separation of tantalum and columbium was effected under optimum conditions. However, this modified technique was not as flexible as the established process, and therefore was not investigated further in large-scale equipment.
Reuse of Solutions
Tests were run to determine the possibility of recycling spent solutions for reuse. As approximately 75 percent of the separation cost is for chemicals; a substantial reduction in separation costs is possible by recycling operations. The four-step process is ideally suited for a recycle-type system. It would be necessary to recover the acid and small amount of tantalum and columbium left in the spent feed solution. The possibility of recycling the aqueous phase from the reextraction of organic was considered, but this solution is dilute in acid and in metal. The metal content was recovered by ammonia precipitation, and the filtrate discarded. Methyl isobutyl ketone must be recovered and reused, because of its high cost. To recover the solvent it was necessary to strip it of its metal content and then scrub it to remove any ammonia salts.
Recycling Feed Solutions
The feasibility of recycling the spent feed solution was investigated. When the feed solution was processed through the coextraction step, 98 percent of the valuable metals and 80 percent of the fluoride introduced as hydrofluoric acid transferred into the organic extract. The spent feed contained 2 percent of the valuable metals, 20 percent of the fluoride, and 100 percent of the sulfuric acid. To regenerate the spent feed, an amount of hydrofluoric acid equal to the fluoride that had transferred to the organic extract was used to dissolve an amount of hydrated tantalum and columbium oxide equal to that used in the initial feed solution. The spent feed solution was then used to dilute the hydrofluoric acid solution for a new feed solution.
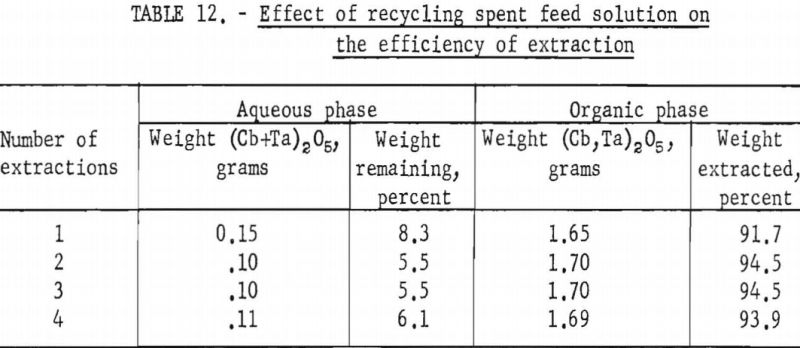
A series of shaker-scale tests was made to determine the effect of recycling the spent feed solution from the initial coextraction operation. Four tests were run simultaneously. In the first run, used as a control, the spent feed was not returned to the system. In the second, third, and fourth runs the spent feed was recycled one, two, and three times, respectively. Fifty milliliters of the initial feed solution contained 5 grams of hydrated tantalum and columbium oxide (Ta:Cb = 1:1) and was 5.6 N in hydrofluoric acid and 9 N in sulfuric acid. The feed solution was then contacted with 100 milliliters of methyl isobutyl ketone. Forty-two milliliters of the resulting spent feed was regenerated by the addition of 8 milliliters of 50 percent hydrofluoric acid in which had been dissolved 5 grams of hydrated tantalum and columbium oxide. This regenerated feed solution was then processed through the initial coextraction step. At the completion of the test, the metals in the spent feed solution and organic extract were precipitated with ammonium hydroxide, ignited, weighed, and analyzed.
Tables 12 and 13 show the results of these tests. The repeated recycling had no deleterious effect on the efficiency of the extraction or on the purity of the products. The reuse of the spent feed increased the efficiency of the extraction by 2 to 3 percent and saved approximately 20 percent of the hydrofluoric acid and 100 percent of the sulfuric acid required for the preparation of the initial aqueous feed solution.
Reconditioning Methyl Isobutyl Ketone
Although the tantalum can be precipitated directly from the organic phase with ammonium hydroxide, the subsequent filtering operation is difficult and time consuming because of the presence of solvent in the precipitate. In addition, some solvent is lost by evaporation because of the heat generated during neutralization.
Small-scale tests were run to develop a method for removing and recovering tantalum compounds from the final organic solutions by stripping them with aqueous solutions. For these tests, samples of the final organic phases from several of the small mixer-settler column runs were shaken with various aqueous solutions, and the two phases were separated. The metals present in each phase were precipitated with ammonium hydroxide. The hydroxides were collected on a filter and ignited at 800° C. Weights of the ignited oxides were used to calculate the efficiency of the stripping procedures. Water and different concentrations of ammonium fluoride were tested as stripping solutions. Ammonium fluoride solutions were prepared by mixing calculated volumes of ammonium hydroxide and concentrated hydrofluoric acid with water to result in 1-, 3-, 5-, and 10-percent weight/volume solutions. Conditions and results of these tests are given in table 14. Use of 5 percent ammonium fluoride as stripping solution effectively removed and recovered the metals from the organic phase and resulted in clean phase separation.
Several tests were made t o determine the most effective procedure for reconditioning methyl isobutyl ketone for reuse. Scrubbing the stripped solvent with water and with 0.5 N H2SO4 caused emulsions to form between the two
phases. Use of 1 N H2SO4 as a scrubbing agent effectively reconditioned the solvent. Scrubbed solvent was reused many times in large-scale tests with satisfactory results.
Rate of Transfer Tests
Before initiating the large-scale investigations, a test was made to determine the rate of transfer of the valuable metals from the aqueous feed solutions to the organic phase. Sufficient aqueous feed solution was prepared to provide eight portions for these rate tests. Each portion was shaken with two volumes of methyl isobutyl ketone. The time of contact was varied from 0.5 minutes to 30 minutes. Table 15 gives the results of these tests.
The results of this test indicate that tantalum and columbium transfer rapidly from the feed solution to the organic phase. Although there are small differences in the percent of metal transferred, the authors feel that these differences are not significant and conclude that contact time of 1 minute is sufficient for transfer.
Large-Scale Tests
The results of small-scale tests indicated that the hydrofluoric acid-sulfuric acid-methyl isobutyl ketone system offered a method of separating columbium from tantalum, as well as from other metallic impurities. The investigations were continued in an effort to devise a procedure and apparatus for continuously separating these metals. Small-scale tests indicated the tantalum and columbium fluoride complexes transferred rapidly. Because equilibrium was reached quickly, the use of pulse column equipment for contacting the two phases was deemed to be superfluous.
Apparatus
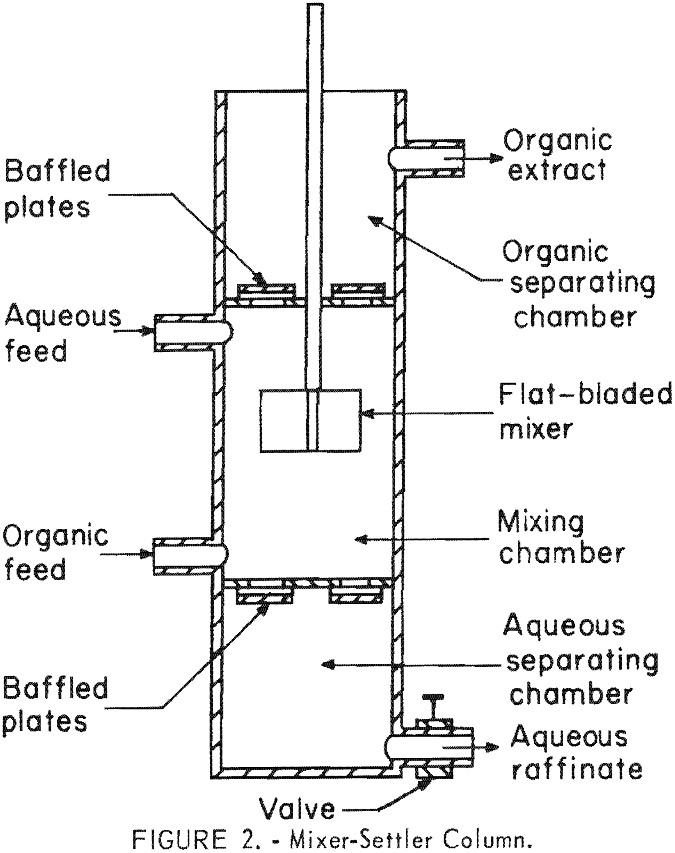
A device, called a mixer-settler column, was designed and used for the initial continuous countercurrent tests. This contactor, shown in figure 2, consists of three chambers: A mixing chamber in the center and settling chambers at the top and bottom. The aqueous and organic liquids were pumped into the mixing chamber and flowed countercurrently to each other, and were intimately mixed by means of a flat-bladed impeller. The solvent phase rose to the upper settling chamber from which it was continuously removed. The heavier aqueous phase settled out into the lower settling chamber from which it was continuously drained. Cylindrical mixer-settler columns of 6-, 8-, 10-, and 12-inch diameters were fabricated of polyethylene. In general, the height of the mixing chamber of each column was twice the diameter, and the height of each settling chamber was approximately 70 percent of the diameter. Baffled plates served to minimize turbulence in the liquids passing from the mixing chamber into the settling chambers.
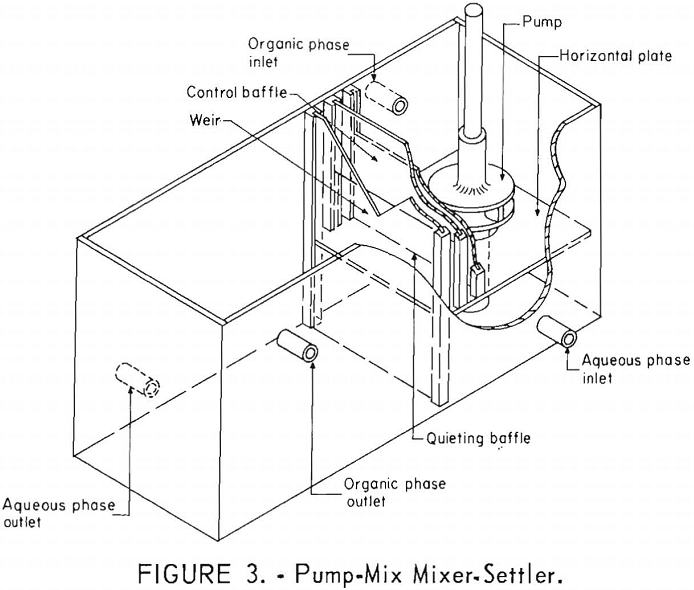
Because the mixer-settler columns retained large amounts of solution, a small version of the pump-mix mixer-settler was fabricated. These units were a modification of the design reported by Webster and Williamson. The pump-mix mixer-settlers, shown in figure 3, were fabricated of polyethylene.
The mixing section was 4 by 4 by 4 inches and was divided by a horizontal plate located 1-½ inches from the bottom. A ¾-inch hole in this plate connected the two chambers in the mixing section. The pump, fabricated of polyethylene, was 1-½ inches in diameter and driven by a variable speed motor. The settling section of the mixer-settler unit was 6 by 4 by 4 inches. A quieting baffle, a control baffle, and a weir served to connect the upper mixing chamber and the settling section. The aqueous and organic liquids were pumped into the mixing section and intimately mixed by the pump. The mixed phases passed over the quieting baffle, under the control baffle, and spilled over the weir into the settling section. Actually, the two phases disengaged in the space between the quieting baffle and the weir and were almost completely separated as they passed over the weir.
Procedure and Results for Mixer-Settler Column
Stepwise Tests, Mixer-Settler Column with Gravity Feed System
The initial large-scale separation tests were made in steps. Each step of the overall process was run continuously, but separately.
In general, the procedure for making a continuous separation run was very similar to that employed for the shaker-scale tests. Aqueous solutions were prepared and held in polyethylene delivery tanks. Fresh or reclaimed solvent was held in a second polyethylene delivery tank. These solutions flowed by gravity into a 6-inch-diameter polyethylene mixer-settler column. The rate of flow was controlled by polyethylene needle valves. In the mixing section of the column, the two phases were mixed by a flat-bladed impeller. After the phases were mixed, they disengaged and flowed out of the column into polyethylene collecting tanks. The aqueous and organic solutions flowing out of the column were sampled periodically, usually at half-hour intervals. A composite
sample of each phase was taken at the end of the run. The metals present in each phase were precipitated with ammonium hydroxide. The hydroxides were collected on a filter, ignited at 800° C weighed, and analyzed. The volume of each phase was measured.
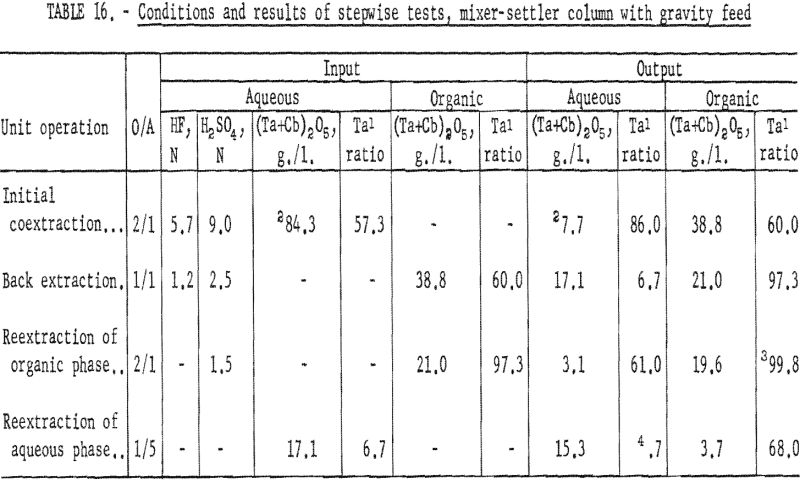
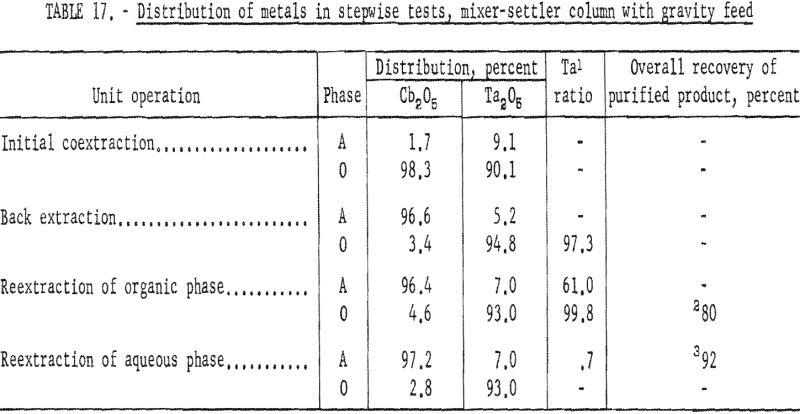
The four-step process developed in shaker-scale tests was used for continuous runs. Runs in the mixer-settler column were made under the same conditions. An aqueous feed solution (Ta:Cb = 3:2) was contacted with solvent in the column to transfer the columbium and tantalum fluoride complexes to the solvent phase. Samples were taken and analyzed. The results of several tests indicated that about 92 percent of the tantalum and columbium was transferred. The aqueous raffinate was discarded and the metal-laden organic solution saved for the back extraction operation.
The procedure for the back extraction was similar. The metal-laden solvent phase was contacted in the same mixer-settler column with a dilute hydrofluoric-sulfuric acid solution to extract columbium preferentially from the organic solution. Approximately 95 percent of the columbium and 5 percent of the tantalum were extracted by the weak acid solution. As in the initial coextraction tests, the aqueous and organic solutions flowing from the column were sampled periodically during the run. A composite sample was taken of each phase when the run was completed. Both the aqueous and organic solutions were saved for further processing.
The tantalum-rich organic phase was again contacted in the same mixer-settler column with a dilute sulfuric acid solution. This reextraction operation was made to remove columbium remaining in the organic phase. The tantalum product in the solvent after this reextraction usually contained less than 0.2 percent columbium.
The columbium-rich aqueous phase was processed in the same column to remove tantalum. This aqueous phase was contacted with a small amount of fresh solvent to extract the tantalum, leaving a columbium product usually containing less than 0.2 percent tantalum. Conditions and results of these tests are given in tables 16 and 17.
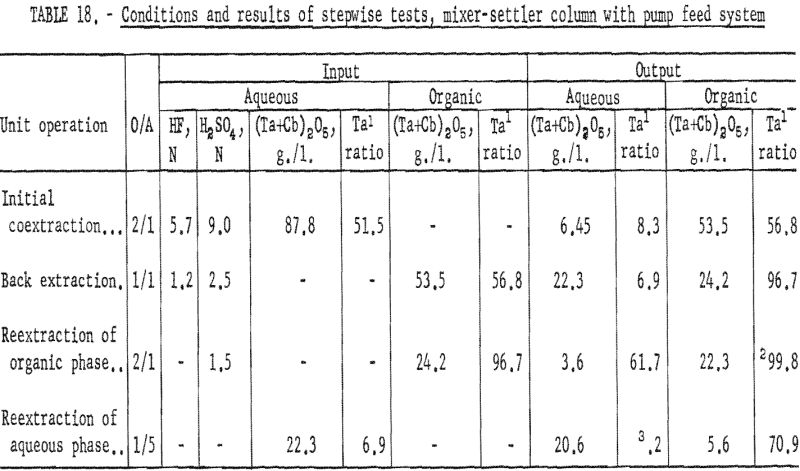
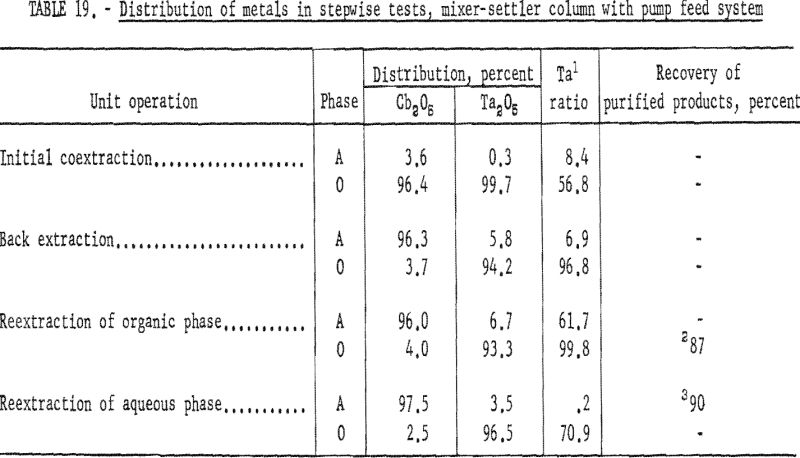
Stepwise Tests, Mixer-Settler Column With Pump Feed system
A second series of stepwise separation tests was made with the mixer-settler column in which the solutions were delivered to the column by proportioning pumps. The gravity feed system did not prove satisfactory because the polyethylene needle valve did not deliver solutions at a constant rate. These runs in which pumps were used to meter the solutions were essentially the same as the gravity-feed tests. The use of pumps to control flow rates resulted in a more accurate determination of conditions necessary for continuous separation. After more tests had been made and more data were obtained, large mixer-settler columns were fabricated and used for runs in which large volumes of solution were processed. Conditions and results of the mixer-settler column tests with pump feed system are shown in tables 18 and 19. Ta:Cb ratio of feed material for these tests was 1:1.
Continuous Runs, Mixer-Settler Columns in Cascade
Two runs were made with mixed tantalum and columbium oxides extracted from a euxenite concentrate. This material contained 15.3 percent moisture. The ignited product contained 83 percent columbium oxide and 12 percent tantalum oxide.
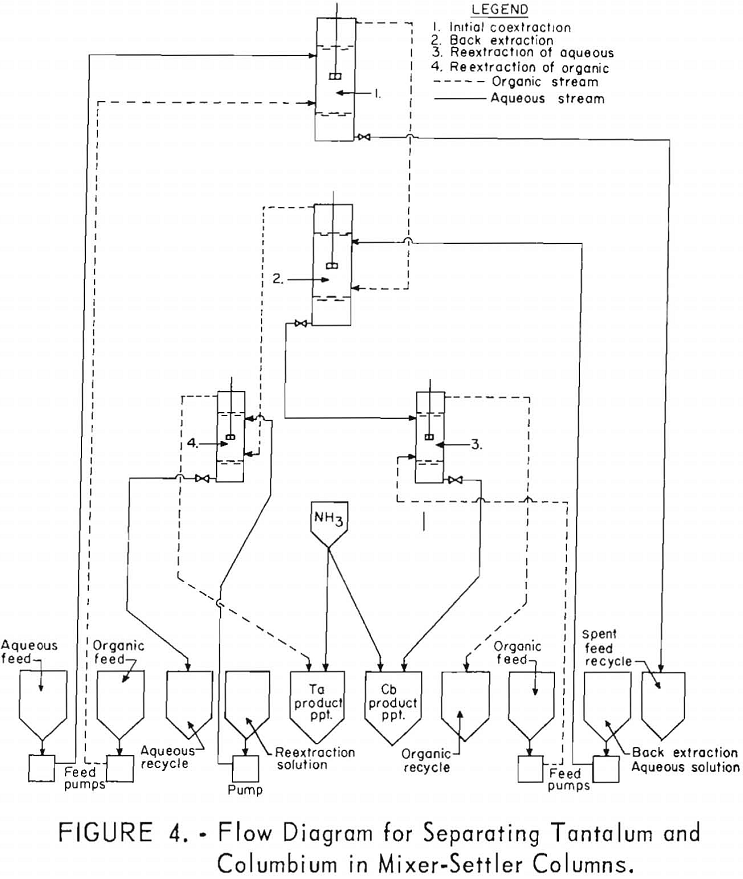
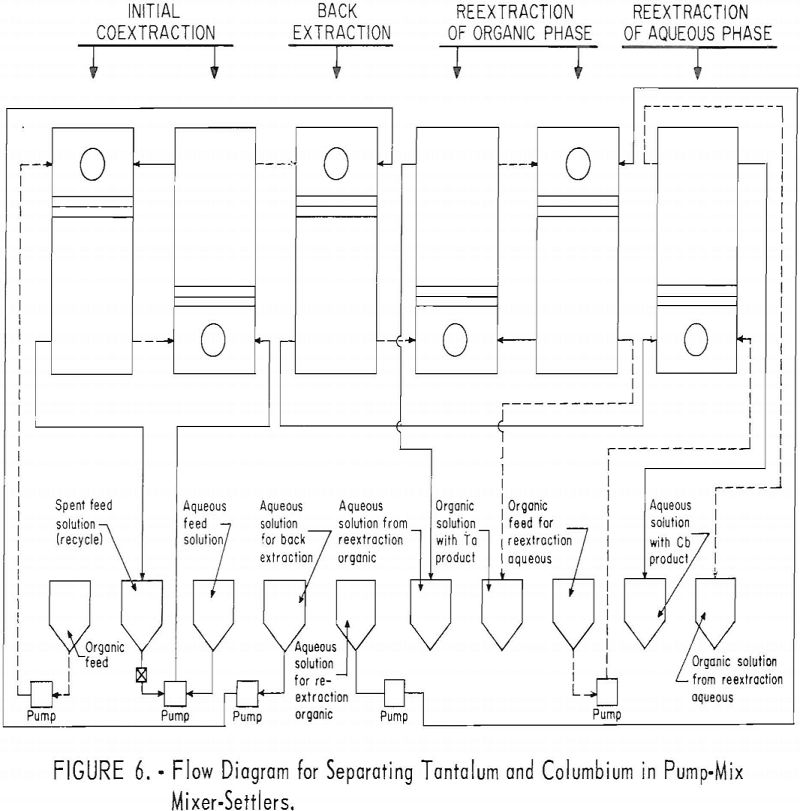
The equipment for this work consisted of four mixer-settler columns fabricated of polyethylene. Solutions were moved from feed tanks to the columns by acid-resistant proportioning pumps. The organic and aqueous streams from the mixer-settler units flowed through the system by gravity. Arrangement of the columns and pumps is shown in figure 4.
Feed solutions were prepared by dissolving the hydrated oxides in concentrated hydrofluoric acid. Sulfuric acid and water were added to the resulting solution to obtain the desired metal and acid concentrations. Two feed solutions were prepared. The first was 5.6 N in hydrofluoric acid, 9 N in sulfuric acid, and contained 82.25 grams of mixed oxides per liter. The second was 5.6 N in hydrofluoric acid, 10 N in sulfuric acid, and contained 72.0 grams of mixed oxides per liter.
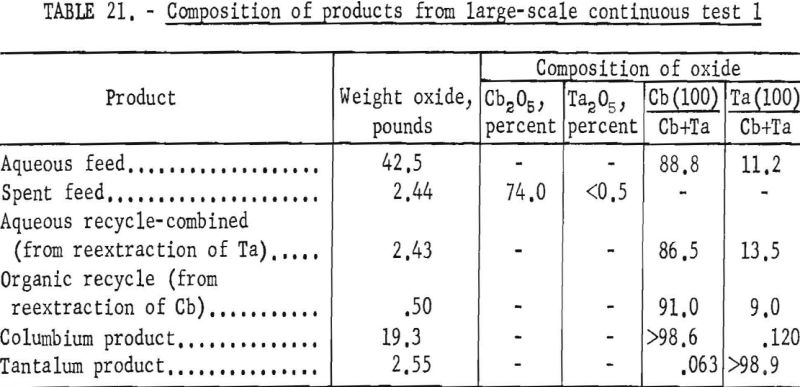
For the initial coextraction operation of the first continuous run; aqueous feed solution and fresh solvent, at an organic-to-aqueous volume ratio of 2:1, were pumped into the uppermost mixer-settler column of a four-unit assembly. After being mixed, the phases disengaged and the metal-laden solvent flowed into the second column while the spent feed was removed from the system. In the second or back-extracting mixer-settler, the solvent was contacted with a dilute hydrofluoric-sulfuric acid solution at a volume ratio of 1:1. The aqueous phase from the back extraction flowed to a third mixer-settler column where it was contacted with fresh solvent in an organic-to-aqueous volume ratio of 1:4. This reextraction operation removed the tantalum impurity, and the aqueous stream from this unit contained the final columbium product. The organic phase from the back extraction operation flowed into the fourth column and was contacted with dilute sulfuric acid in an organic-to-aqueous volume ratio of 2:1. The solvent stream from this operation contained the tantalum product that was contaminated with 1 percent columbium. This solvent had to be contacted a second time with dilute sulfuric acid in an organic-to-aqueous volume ratio of 2:1 to obtain a satisfactory tantalum product. During the run, samples were taken periodically of the spent feed solution from the initial coextraction and of the columbium product and tantalum product streams. When the run was completed, samples of composite solutions were collected. Metal concentrations of the solutions were determined by ammonium hydroxide precipitation. Composition of the resulting oxides was determined by spectrographic analyses. Operating conditions for this run are given in table 20.
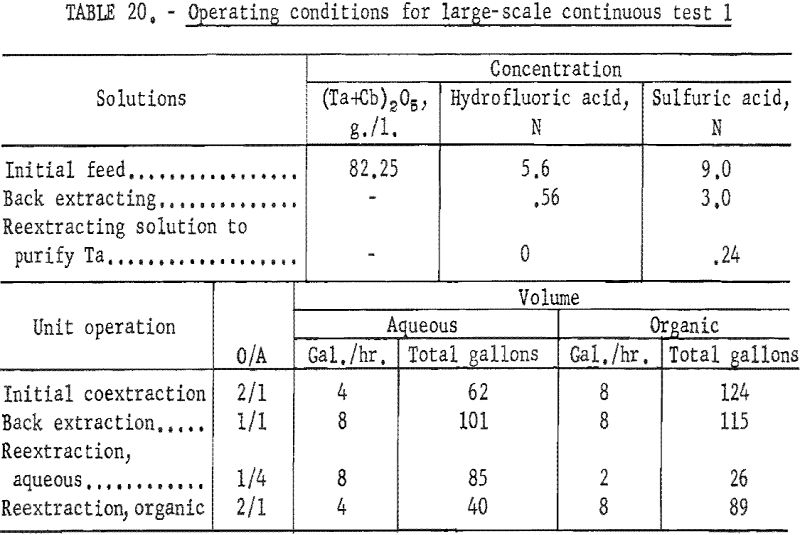
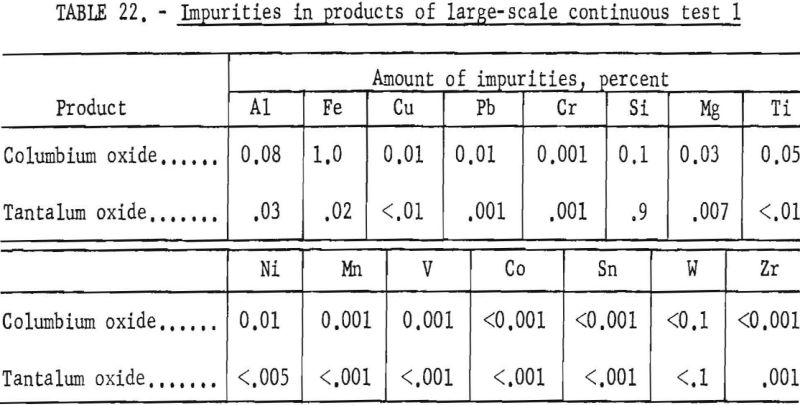
Table 21 shows the weights and the tantalum-columbium composition of the intermediate and final products from this run. Table 22 shows the metallic impurities in the final products. Some impurities were introduced by use of technical grade hydrofluoric acid. The 70 percent hydrofluoric acid used to make up the aqueous solutions, except the ammonium fluoride stripping solution, contained 3.14 grams of metal per liter, of which 1.9 grams per liter was iron. The columbium product was precipitated from the aqueous solution with ammonium hydroxide and dewatered by means of a Moore leaf filter. The tantalum product was stripped from the solvent with a 5-percent ammonium fluoride solution. Ammonium hydroxide was added to the stripping solution and the precipitated tantalum product was collected on a filter.
Recovery of 79.5 percent of the columbium and 86.6 percent of the tantalum was achieved. These values were calculated by dividing the weight of the final product by the weight of the columbium or tantalum oxide that was removed from the separation system during the run. The amounts of oxides that remained in the mixer-settler columns at the completion of the run were not included with the weight of tantalum or columbium oxide that was used as the divisor in the recovery calculations. This operation was a short-term run; the length of the run was limited by the volume of feed solution. When all the feed solution was added, the run was stopped, and significant volumes of metal-bearing solutions were left in the mixer-settlers. None of the recycle solutions was returned to the system. The flow diagram in figure 4 shows that the solutions that could be recycled are the spent feed solution from the initial coextraction operation, the organic product from reextraction of the aqueous phase, and the aqueous product from reextraction of the organic phase. Calculations indicated that if these solutions were recycled through the separation system at the efficiency calculated from the results of the short term runs, the recovery for a long term-run would be 96 percent for columbium and 98 percent for tantalum.
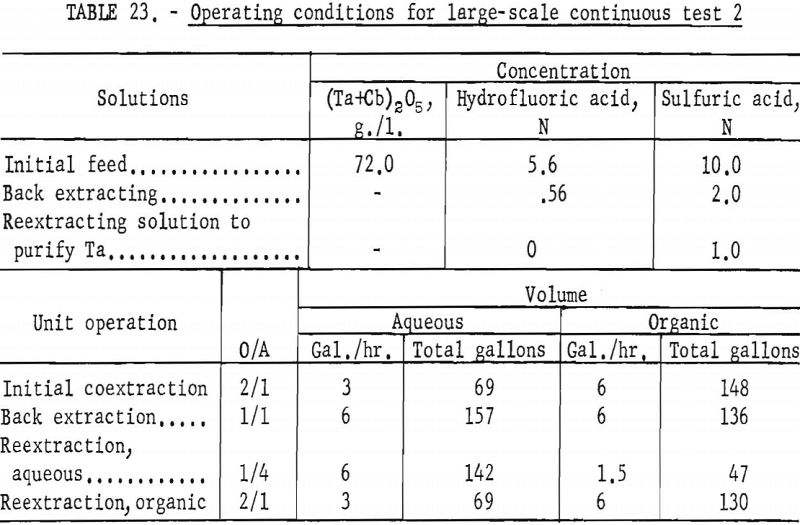
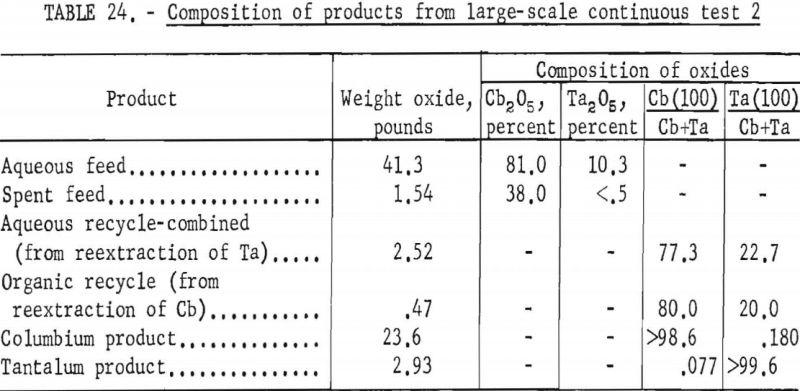
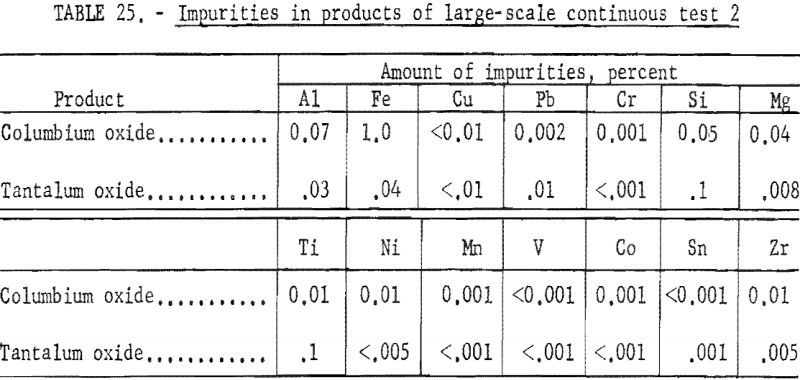
The apparatus and procedure used for the second run were the same as those described for the previous run. However, conditions of acid concentration and flow rates were changed to effect a higher recovery of the columbium product (see table 23). To eliminate the stripping operation, the final tantalum product was precipitated directly from the organic solution, and collected on a filter. Results of this run are given in tables 24 and 25.
Recoveries of 85.9 percent columbium and 80.4 percent tantalum were calculated. Again these figures were based upon the weight of tantalum or columbium oxides that passed through the system, excluding the amounts of oxides that were left in the mixer-settler columns at completion of the run. If recycle solutions had been returned to the separation system, as they would have been in a long run, the estimated recoveries would be 98 percent columbium and 96 percent tantalum.
Procedure and Results of Pump-Mix Mixer-Settler Tests
Tests were made to obtain operating data for single- and two-stage pump-mix mixer-settler units. Batch tests were made through the four steps of the tantalum and columbium separation process.
Stepwise Tests with Single- and Two-Stage Units
In a single-stage unit, the aqueous solution is introduced into the bottom of the mixing section; the solvent enters the top of the mixing section. The aqueous solution is then lifted by the mixer pump and dispersed through the organic phase. The two phases then pass through the weir into the settling chamber. In the settling chamber they separate and the lighter organic phase becomes the top layer; then both phases are removed for further processing.
The two-stage pump-mix mixer-settler unit in effect is two single-stage units in series, solutions flowing countercurrent to each other. As in single-stage units, the aqueous solution enters the bottom of the first unit mixing chamber, and the organic phase from the second unit settling chamber enters the top of the first unit mixing chamber; these phases are mixed and flow to the first unit settling chamber; they separate, and the organic phase is removed for further processing, The aqueous phase flows from the first unit settling chamber to the bottom of the second unit mixing chamber. Fresh organic is introduced into the top of the second unit mixing chamber. The phases are mixed and flow to second unit settling chamber. The phases separate; the lighter organic phase flows to the top of the first unit mixing chamber, and the aqueous is removed for further processing.
Three control methods were used to maintain the organic-to-aqueous ratio: The pumping and mixing speeds of individual units, the rate at which solutions were introduced into the system, and the rate the phases were removed.
A batch of five gallons of feed solution was processed through the separation system using single-stage units; secondly, five gallons of feed were processed using a two-stage pump-mix mixer-settler unit. The feed solution processed through the single-stage units contained 74.0 grams of hydrated tantalum and columbium oxide per liter, and was 5.6 N in hydrofluoric acid and 9 N in sulfuric acid. In the initial coextraction step, the feed solution was contacted with solvent in an organic-to-aqueous volume ratio of 2:1. The phases from the initial coextraction step were collected in polyethylene containers. The aqueous phase could be regenerated by the addition of a hydrofluoric acid solution of hydrated tantalum and columbium oxides. The organic phase from the initial coextraction step was contacted in the back extraction step with an aqueous solution of 1.12 N hydrofluoric acid and 2 N sulfuric acid. The volume ratio of aqueous-to-organic for the back extraction step was maintained at 1:1. These phases were collected in polyethylene containers. The organic phase from the back extraction step was contacted in the reextraction step with a 1 N sulfuric acid solution. The organic-to-aqueous volume ratio was 4:1. These solutions were collected, and the contained metals were precipitated with ammonium hydroxide and collected on a filter. The aqueous phase obtained from the back extraction step was reextracted with one-fifth its volume of fresh solvent. The metals contained in the resulting aqueous and organic phases were precipitated with ammonium hydroxide, collected on a filter, and dried. The organic phase can be recycled into the system.
The feed solution processed through two-stage pump-mix mixer-settler units contained 67.4 grams of hydrated tantalum and columbium oxide per liter of solution; the solution was 5.6 N in hydrofluoric acid and 9 N in sulfuric acid. The extraction and separation solutions and volume ratios were the same for the run made in the two-stage units as they were for the single-stage unit run.
These tests, comparing the two kinds of mixer-settler units, showed that the two-stage unit was more efficient than the single-stage unit for the initial extraction operation and for the organic reextraction step. In the initial coextraction step with the two-stage mixer-settler unit, more than 99.8 percent of tantalum and columbium compounds in the initial feed solution transferred to the organic phase. In the single-stage unit, 96.1 percent of the mixed oxides transferred to the organic phase. In the reextraction of the tantalum-rich organic phase, use of the two-stage mixer-settler decreased the columbium content of the tantalum product from 5.8 to less than 0.2 percent, and increased the recovery of tantalum from 67.8 to 83.8 percent. The advantage of the two-stage unit over a single-stage unit was not as apparent in the back extraction step, nor in the reextraction of the columbium-rich aqueous phase. Table 26 compares the results obtained with single-stage and two-stage units through the four steps of the process.
Continuous Test, Pump-Mix Mixer-Settler Units in Array
A continuous ran was made by using the array of six pump-mix mixer-settlers (see figure 5). These units were arranged as shown in figure 6. The initial coextraction step used two pump-mix mixer-settler units; the back extraction step used one unit; the reextraction step of the columbium rich aqueous phase used one pump-mix mixer-settler unit; and the reextraction of the tantalum rich organic phase used two pump-mix mixer-settler units.
For the continuous run, 10 gallons of initial feed solution were prepared by dissolving hydrated tantalum and columbium oxide in 50-percent hydrofluoric acid. This solution was allowed to cool to room temperature and was then treated by the addition of concentrated sulfuric acid. The resulting solution was cooled and filtered; sufficient water was added to obtain the desired 10 gallons of feed solution. This feed solution contained 45.8 grams of tantalum-columbium oxide per liter; the Ta:Cb ratio was 2:3. The initial feed solution was processed through the array of pump-mix mixer-settler units. There was no interruption between steps. Aqueous-to-organic volume ratios throughout the process were maintained by controlling the pumping speeds. Results of this run are shown in tables 27 and 28.
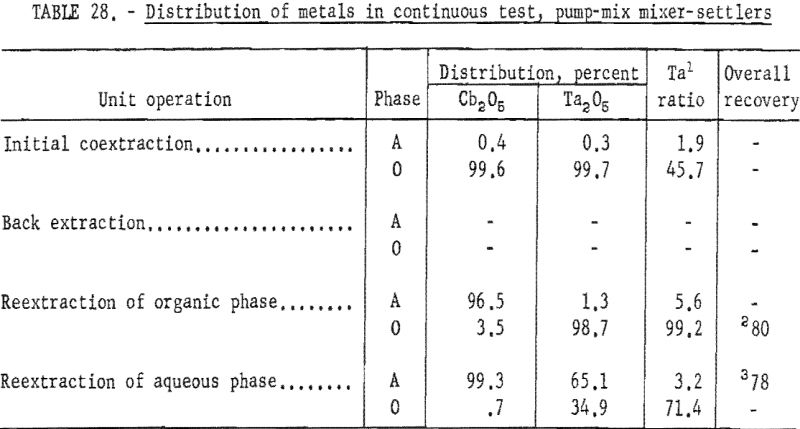
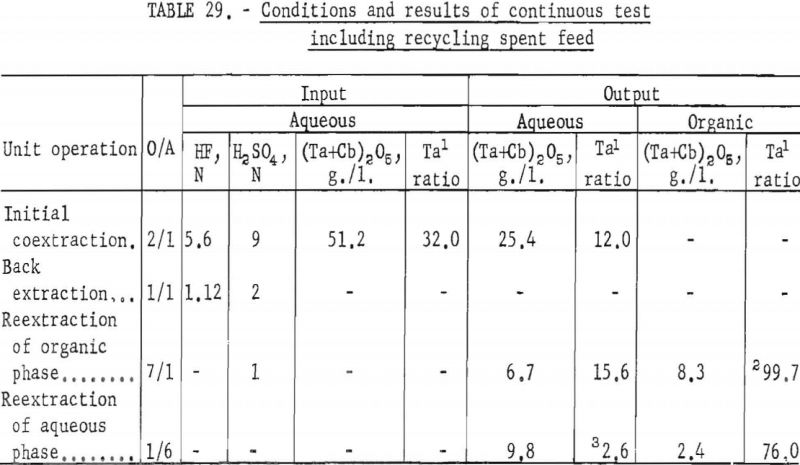
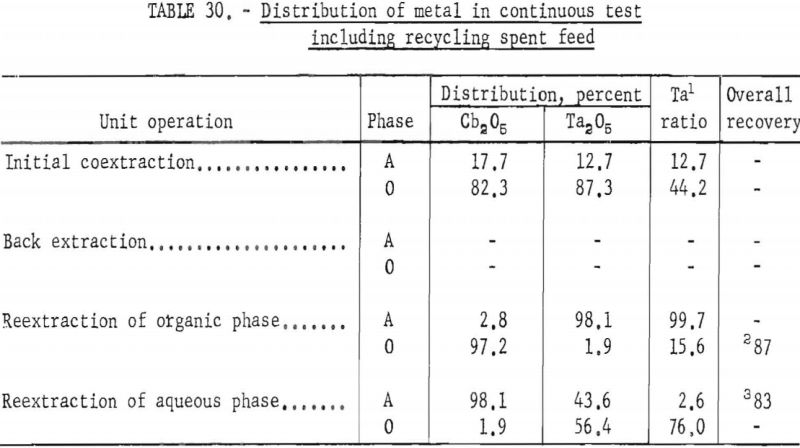
Continuous Test, Recycling Aqueous Feed Solution
One continuous run to study the recycling of the spent feed solution was made by using the same array of pump-mix mixer-settler units. The regenerated feed solution was prepared by dissolving hydrated tantalum and columbium oxide in 50 percent hydrofluoric acid. Six and seven-tenths gallons of regenerated feed solution was obtained by adding sufficient spent feed solution to the hydrofluoric acid-hydrated oxide solution. The regenerated feed solution was then filtered and processed through the pump-mix mixer-settler units. The metering pumps used for this test were very erratic at low pumping speeds. As a result, organic-to-aqueous ratios for both reextraction steps were lower than desired.
In this one test the results show that although less than 90 percent of the columbium and tantalum was transferred to the organic phase during the initial coextraction, processing losses through the rest of the operation were low. The overall recovery of purified columbium and/or tantalum was greater than that achieved with mixer-settler columns. Tables 29 and 30 show the results of this run.