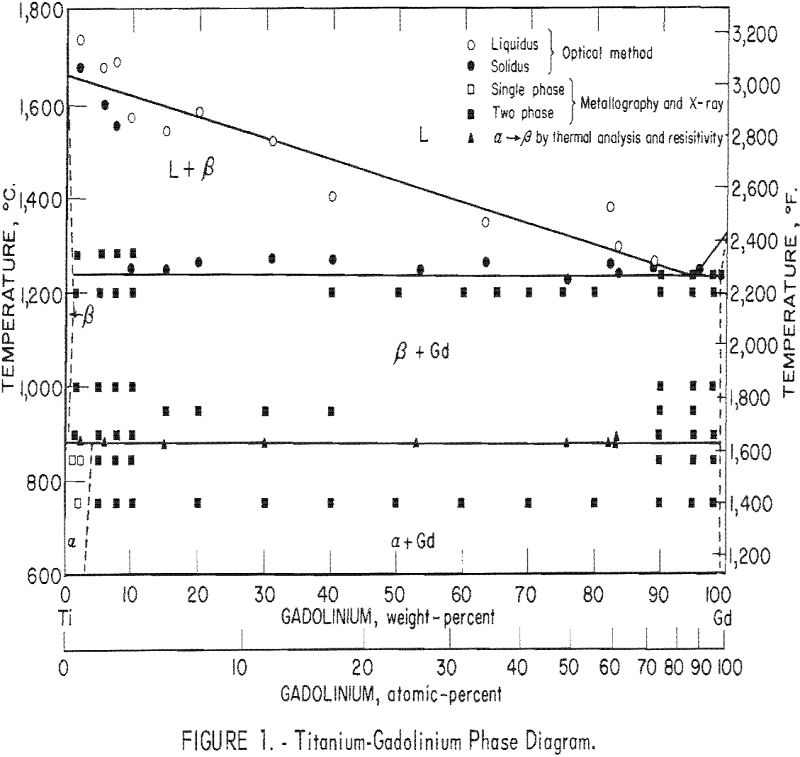
The results of this investigation indicate that the titanium-gadolinium phase diagram is composed of a single eutectic reaction and a peritectoid reaction at the alpha-beta transformation. The eutectic isotherm has been established at 1,240 ± 10° C. and extends from approximately 1 pct. gadolinium to beyond 99 pct. gadolinium at this temperature. The solid solubility of gadolinium in beta-titanium appears to decrease slightly with decreasing temperature to 885 ± 5° C. At this temperature the peritectoid reaction occurs with the peritectoid horizontal extending throughout the two-phase field. Solid solubility of gadolinium in alpha-titanium below this reaction increases to approximately 4 pct. The limit of solid solubility of titanium in gadolinium has not been determined precisely, but it is apparently less than 1 pct.
Mechanical properties based on hardness measurements show little change when gadolinium is added to titanium. Likewise, only a small increase in hardness is obtained by heat treatment. It is doubtful that effective improvements in mechanical properties can be obtained by solid solution hardening in this system.
Alloys containing less than 10 pet. gadolinium can be cold-worked extensively, whereas only moderate degrees of cold-working are obtained at higher gadolinium contents. All alloys can be hot-worked at 750° C. by sheathing in iron. Observations indicate that the machinability of all alloys is good.
Corrosion resistance of these alloys is expected to be poor on the basis of results experienced on etching metallographic specimens. The addition of gadolinium appears to cause a marked decrease in corrosion resistance. This is especially true of the gadolinium-rich alloys.
The high thermal-neutron cross section of gadolinium and the good mechanical and corrosion properties of titanium suggest that alloys of these two metals might be useful as control rod material. To aid in establishing the feasibility of this combination, the Federal Bureau of Mines investigated the titanium-gadolinium system. The results indicate that the system is composed of a single eutectic reaction and a peritectoid reaction involving the alpha-beta transformation of titanium. The eutectic isotherm extends from 1 to 99 pct. gadolinium at 1,240° C. The established eutectic composition is about 95 pct. gadolinium. The peritectoid reaction occurs at 885° C. and extends almost over the entire composition range. Hardness increases slightly with additions of gadolinium, and little change is obtained by heat treatment. Alloys containing less than 10 pct. gadolinium are amenable to extensive cold-working, but alloys having higher percentage of gadolinium content are only moderately cold-workable. All alloys can be hot-worked in iron sheathing at 750° C. Observation indicates that all compositions possess good machinability. Corrosion resistance to water at room temperature is apparently poor, especially in the gadolinium-rich alloys.
Introduction
A combination of the good mechanical and corrosion properties of titanium and the variety of thermal-neutron cross sections characteristic of the rare-earth metals suggests that alloys of these metals may be used for control rod materials. For this reason the Bureau of Mines proposed a study of the titanium-gadolinium alloy system, and the Atomic Energy Commission sponsored the investigation.
Although the phase diagrams of titanium alloyed with the more common metals are relatively well established, little information is available on titanium alloyed with any of the rare-earth metals. A literature search conducted at the beginning of this project revealed no information on titanium-gadolinium alloys. During the investigation some helpful data on titanium-rich alloys were published by other investigators.
To establish the suitability of these alloys, it is necessary to determine alloying characteristics, fabrication procedures, mechanical properties, and corrosion resistance. This can best be accomplished by establishing the phase diagram for the system and supplementing it with the necessary testing.
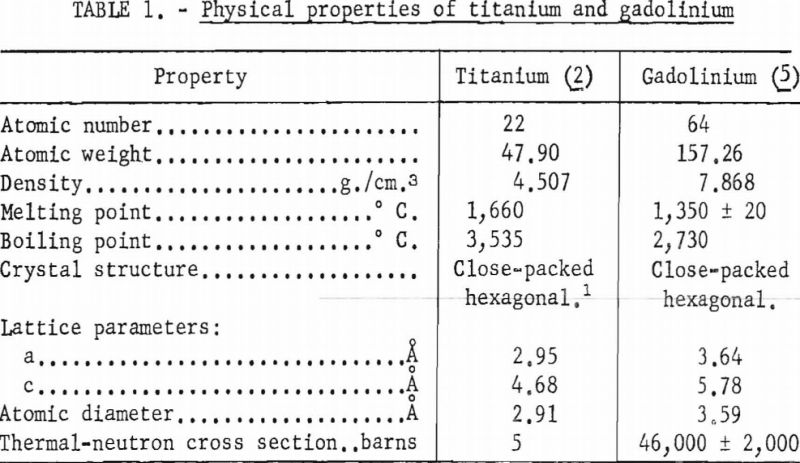
In table 1 selected properties of titanium and gadolinium are presented for reference purposes. A brief inspection of these properties reveals that the size factor is not favorable for extensive solid solubility although the crystal structures are compatible below 882° C.
Experimental Procedures and Results
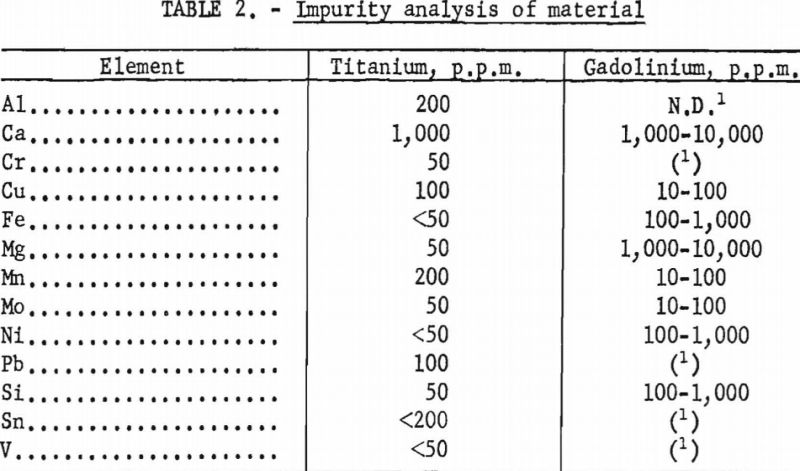
Gadolinium of 99+ pet, purity and electrolytic titanium were used to prepare the alloys of this system. Impurity analyses indicated the presence of several elements. Results of these analyses are given in table 2. With the exception of calcium and magnesium, most of the impurities were negligible in quantity. However, these elements were assumed to have little effect on the phase relations since their low boiling points would permit volatilization during melting. This assumption was confirmed by subsequent analyses of melted alloys in which the concentration of calcium and magnesium was less than 400 and 50 p.p.m., respectively.
The alloys were prepared by melting 30- to 50-g. charges in a nonconsumable, tungsten-electrode arc furnace. The furnace was evacuated to 15 µ, Hg or less, then backfilled with helium to atmospheric pressure. The pressure was again reduced to 15 µ, Hg, and the furnace was backfilled with helium to 5 p.s.i.g. Under this atmosphere the charges were melted into button shapes on a water-cooled, copper hearth. Each button was melted four times and inverted between melting. On the final melting, some of the alloys were suction-cast into a water-cooled, copper mold using a method similar to that employed by Kuhn. Casting in this manner produced an ingot approximately one-fourth inch in diameter by three inches long. Metallographic examination of the cast ingot revealed a small grain size and less segregation than in the button ingots.
Previous experience with zirconium-rare-earth metal alloys indicated that segregation in as-cast material hindered metallurgical analysis. Therefore, most of the alloys were subjected to a homogenization treatment. This treatment consisted of sheathing the ingot in iron pipe and hot-swaging it at 750° C. In this manner the ingot was reduced to a rod approximately one-eighth of an inch in diameter. Surface contamination was removed by centerless grinding.
X-ray fluorescence, spectrographic, and chemical analyses of specimens in the hot-swaged form indicated some variation of gadolinium content in alloys of the same nominal composition. Some losses apparently resulted from volatilization during melting. Copper content increased to approximately 2,000 p.p.m. in some of the gadolinium-rich alloys, but iron content remained essentially constant for all alloys swaged at 750° C. The increase in copper is attributed to melting on the copper hearth.
Initial studies included the determination of machinability, as-cast hardness, and amenability to cold working. The buttons were prepared for hardness testing and rolling by machining flat, parallel surfaces on each side. Observations made in this operation indicated that all alloys were readily machinable.
Hardness tests revealed a slight increase in hardness with the addition of gadolinium. Hardness determinations were also made on alloys subjected to different treatments. These values are presented in table 3.
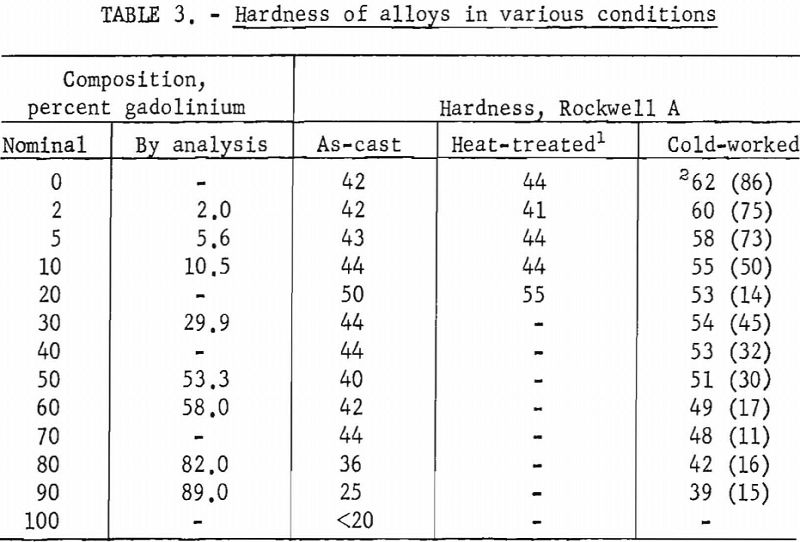
Alloys containing gadolinium up to 10 pct. could be cold-worked extensively. Alloys containing more than 10 pct. gadolinium showed more variability in the extent of cold-working, with the amount of reduction ranging from 11 to 45 pct.
The phase diagram was compiled from results obtained by melting-point determinations, X-ray diffraction analysis, thermal analysis, resistivity measurements, and metallography. The proposed diagram is presented in figure 1, and the results are tabulated where applicable.
Since the alloys reacted with all known crucible material, the solidus and liquidus temperatures were determined by means of the thermal gradient technique using optical methods. In this method a small cylindrical specimen was mounted vertically on a tungsten pedestal. The specimen had a small hole drilled in the upper end to a depth of approximately one-quarter in inch. The entire assembly was suspended inside a tantalum cylinder positioned in the heating zone of a vertical induction furnace, and the tantalum tube therefore served as a heater. The position of the pedestal was adjusted to obtain a negative temperature gradient from specimen to pedestal. As the specimen was heated, the bottom of the hole was observed through an optical pyrometer. Because melting changes the geometry of the bottom of the hole, the first evidence of such a change indicated the solidus temperature. Further heating eventually caused collapse of the specimen and an indication of the liquidus temperature. Results of these determinations are given in table 4.
The effect of gadolinium additions on the alpha-beta transformation of titanium was investigated by using differential thermal analysis and electrical resistivity methods. The results of these two methods agreed reasonably well and are shown in table 5.
Several alloys, homogenized at 950° C. and furnace-cooled, were analyzed by X-ray diffraction. In the range covered, two phases were identified; these were alpha-titanium and gadolinium. However, some additional weak lines were observed and could not be identified. The intensity of this pattern did not appear to vary with composition, and the pattern was probably caused by an impurity phase. A summary of these results is given in table 6.

A considerable number of specimens were analyzed by metallography. Several specimens were examined in the as-cast condition, but most were homogenized and heat treated. Standard polishing techniques were used on the titanium-rich alloys, and these specimens were etched with a solution consisting of 45 ml. HNO3, 10 ml. HF, and 45 ml. H2O. Preparation of the gadolinium-rich alloys presented more difficulty. Several techniques were attempted, but best results were obtained by finish-polishing on diamond wheels lubricated with kerosene. Etching was accomplished best by using a dilute solution of nitric acid in absolute methanol. Any presence of water during final polishing and etching resulted in corrosion as evidenced by a bluish film and pits that formed on the specimen surface.
A cursory examination of as-cast material revealed a definite two-phase structure in alloys containing 10 to 90 pct. gadolinium with evidence of a eutectic structure near 90 pct, gadolinium. However, segregation was severe, and further examination was restricted to hot-worked material that had received a homogenization treatment. Homogenization was accomplished by heating the hot-worked alloys in helium-filled quartz capsules. These were heated to the desired temperature, held for 1 to 24 hr, depending on the temperature, and quenched in water. Alloys were heat-treated by this method at 1,200°, 1,000°, 900°, and 750° C. Examination of the alloys heat-treated at 1,200° C, indicated a two-phase structure extending from 7-½ to 98 pct. gadolinium.
A series of alloys containing 2, 5, 7.5, and 10 pct, gadolinium were heat-treated at 1,280°, 1,200°, 1,000°, 900°, 850°, and 750° C. for determination of the solid solubility of gadolinium in titanium. Metallographic examination of these indicated that two phases were present, although the 2-pct. gadolinium alloy was questionable. A similar treatment of alloys containing 90, 95, and 98 pct. gadolinium revealed two-phase structures.
In an effort to determine solid solubility limits, two new series of alloys were prepared. The titanium-rich alloys contained 0.2, 0.5, 1.0 and 1.5 pct, gadolinium. Examination of these alloys heat-treated at 1,200° and 850° C. indicated that approximately 1 pct. gadolinium would dissolve in beta-titanium at 1,200° C. and that 4 pct. would dissolve at 850° C. in alpha-titanium. Examination of alloys containing 90, 95, 96, 98, and 99 pct. gadolinium, quenched from 1,240° C., revealed two-phase structures and evidence of incipient melting. This would indicate that less than 1 pct. titanium will dissolve in gadolinium.
To better establish the eutectic composition, a technique was devised to allow a specimen to cool slowly from the molten state without contamination. The method consisted of supporting a swaged alloy rod vertically in a small induction furnace using a helium atmosphere. The end of the rod was positioned in the heat zone and allowed to reach the molten state by gradually increasing power. Surface tension prevented the molten drop from falling, and as power was reduced slowly, the end of the rod solidified. A similar treatment was performed in a small electron-beam furnace, and better results were obtained because of the narrow heating zone in this type of furnace. This procedure was used for alloys containing 80, 90, 95, and 98 pct. gadolinium. Metallographic examination of the molten zone established the eutectic composition between 90 and 95 pct.
Discussion of Results
The preparation of alloys by methods used in this investigation was satisfactory. Although the impurity analysis indicated considerable amounts of calcium and magnesium, the melting technique reduced these to a negligible level but increased the copper content. However, this amount of copper should have little effect on phase relations. Suction casting of these alloys was satisfactory and aided in reducing as-cast segregation.
The fabrication of cast material required several different techniques. Although limited operations were made, all alloys appear to possess good machinability. Cold-working cannot be used for forming alloys containing more than 10 pct. gadolinium. Alloys containing less than this amount of gadolinium exhibit a marked increase in hardness when cold-worked. Hot-swaging or rolling in iron sheaths gave good results for alloys containing gadolinium in excess of 10 pct.
Liquidus temperatures determined by the thermal-gradient technique are subject to some variation. The nature of the method used requires the judgment of the operator as well as the realization that black-body conditions are lost as melting progresses and the specimen collapses. Under these conditions there must necessarily be some approximation. However, the results are consistent enough to indicate the liquidus trend and do agree with those observed by metallography.
Solidus temperatures determined by this same method are more reliable. This is especially true where considerable liquid is formed on heating through the eutectic isotherm. At compositions near the terminal solid solutions, less liquid is formed and therefore there is more chance of missing the first indications of melting. This is evident from examining the titanium-rich end of the diagram where several solidus temperatures appear in the neighborhood of the liquidus.
The solidus temperatures as determined by the thermal gradient technique indicate an eutectic isotherm at 1,260° C, However, metallographic examination reveals some incipient melting as low as 1,240° C. The reason for the discrepancy is believed to be the inherent lag in observing changes in the sight-hole geometry with the optical pyrometer. Also possible is the formation of metal vapors near the melting temperature, which would increase the absorption and cause an error in observed temperature.. Under these circumstances the authors believe it justifiable to place the eutectic isotherm below that observed by optical methods. For this reason the eutectic isotherm is established at 1,240 ± 10° C.
The alpha-beta transformation of titanium as determined by resistivity and differential thermal analysis methods seems to occur at the constant temperature of 885 ± 5° C. Results of these two methods compare favorably and indicate essentially no difference in transformation temperature from that of pure titanium. The limits of accuracy of these two methods make it impossible to discern whether this reaction should be identified as peritectoid or eutectoid. The nature of this reaction must then be decided on the basis of solid solubility above and below the alpha-beta transformation.
Solid solubility limits were determined by metallographic examination. The solubility of gadolinium in beta-titanium at 1,240° C, was estimated to be 1 pct. The solubility seemed to decrease slightly with temperature down to 900° C. The solubility of gadolinium in alpha-titanium was estimated to be 4 pct indicating that the solid state reaction is a peritectoid type.
A study of metallographic specimens in the gadolinium-rich region revealed no evidence of solid solubility of titanium in gadolinium. However, this investigation extended to only 1 pct, titanium, and no analysis was conducted below this composition.
All of the solid solubility determinations were hampered by the presence of impurities in the microstructure. Although composition analyses indicated a low impurity content and little increase from melting, metallographic examination revealed some impurity phases in the titanium and gadolinium-rich regions. This observation was supported by X-ray diffraction results where several weak unidentified lines were reported. Although these impurity phases caused difficulty in solid solubility determination, their effect on the diagram was considered negligible.
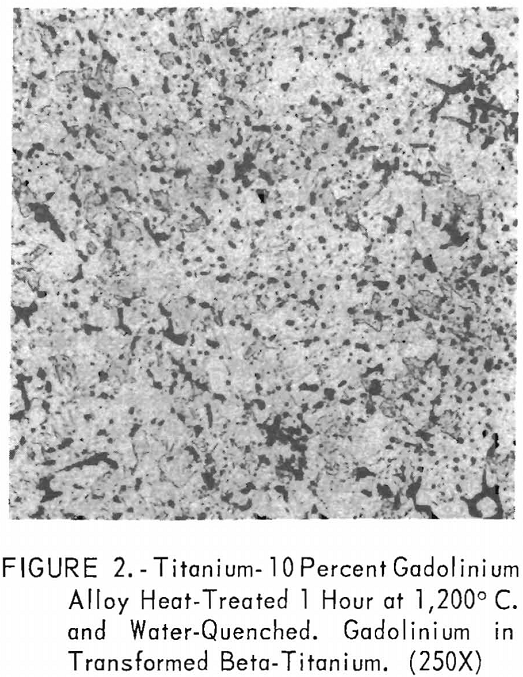
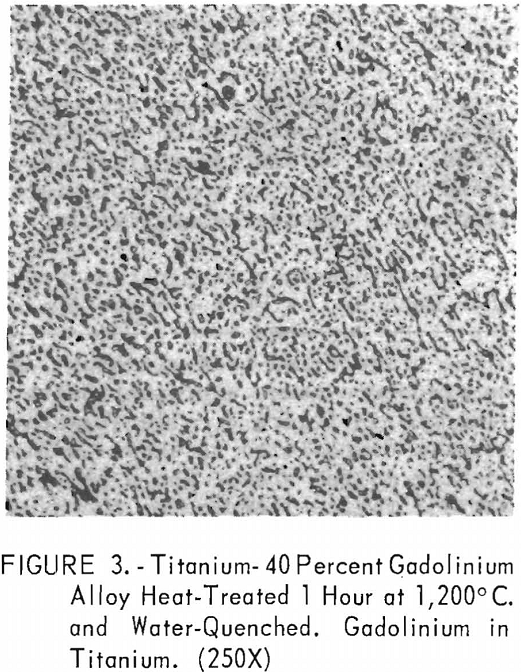
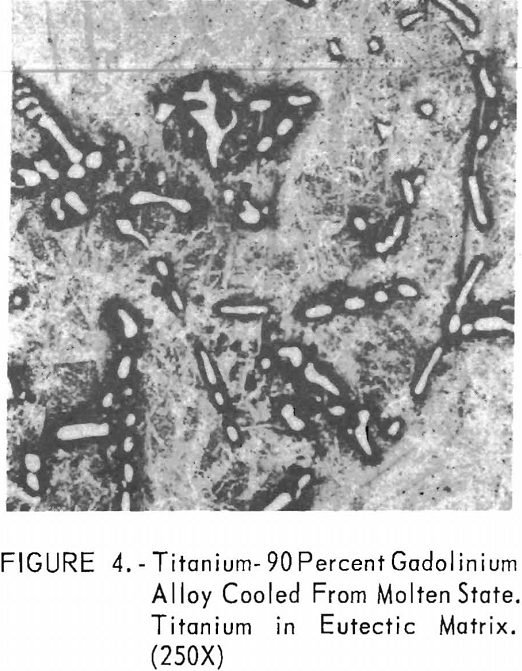
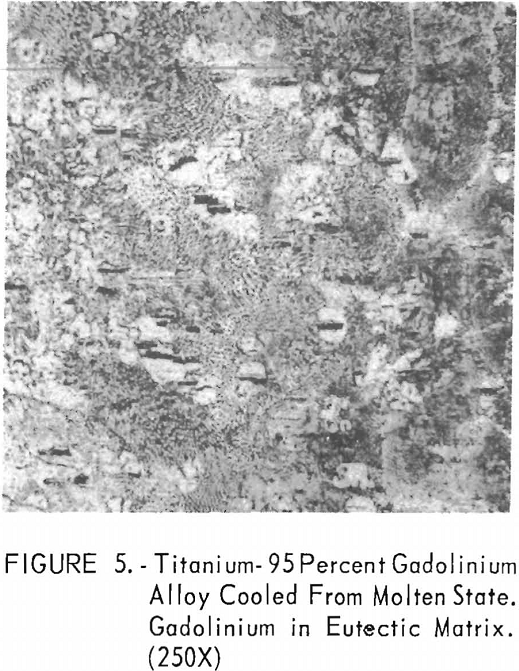
Metallographic examination was also used to correlate the results of other methods. Figure 2 shows the structure of an alloy containing 10 pct. gadolinium quenched from 1,200° C. The gadolinium was probably dispersed in the transformed beta-titanium. As the gadolinium content is increased, the structure shows a proportionate increase in the gadolinium phase as evidenced in figure 3. The structure of an alloy just below that of the eutectic composition is shown in figure 4. This specimen was cooled slowly from the molten state, and a small amount of titanium appeared in the eutectic matrix. Figure 5 indicates the hypereutectic structure at a composition of 95 pct. gadolinium. Here again, the alloy was cooled slowly from the molten state, and small spheroids of titanium appeared dispersed in the gadolinium to form the eutectic structure. Primary gadolinium also was present.
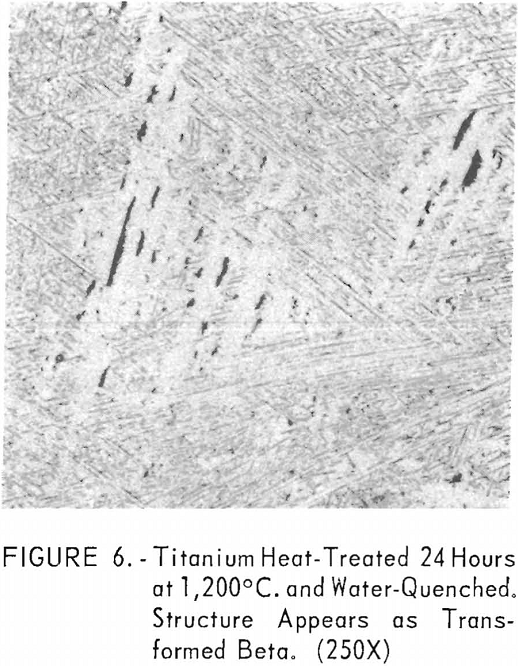
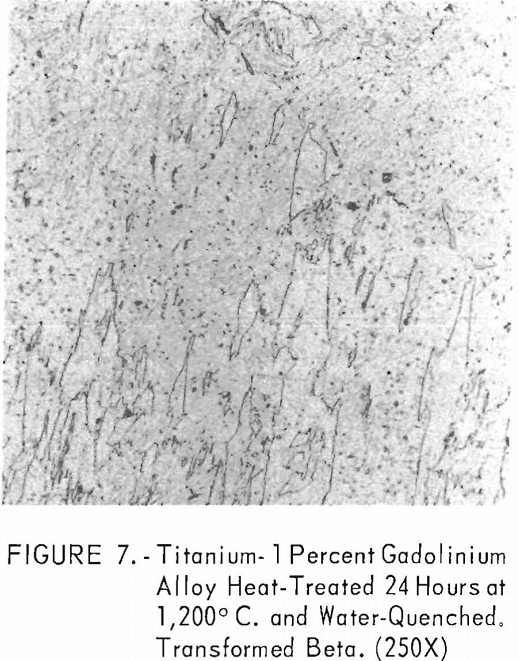
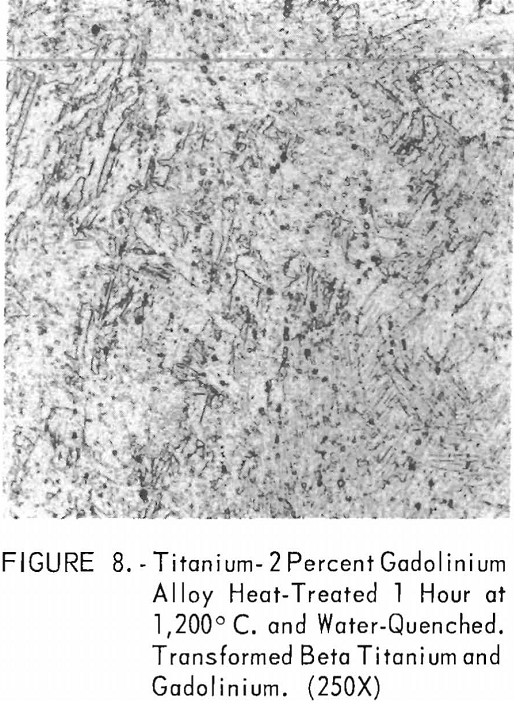
Figures 6 through 8 give an indication of the extent of solid solubility at 1,200° C. of gadolinium in beta-titanium. Figure 6 represents the structure of titanium quenched from 1,200° C. The entire structure was transformed beta-titanium with some evidence of impurities. The addition of 1 pct. gadolinium causes the Widmanstatten structure to disappear. (See figure 7.) The structure is transformed beta-titanium with some evidence of a second phase. This second phase is presumed to be caused by impurities, as no appreciable increase in amount is observed. Figure 8 represents the structure of 2 pct. gadolinium in titanium, which is definite evidence of the gadolinium phase.
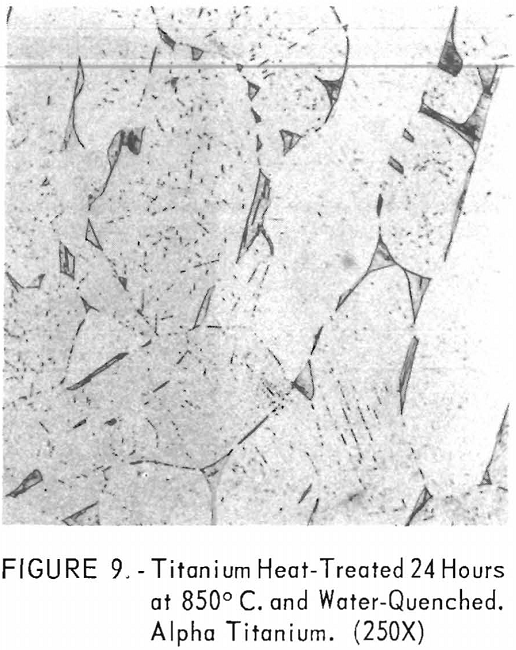
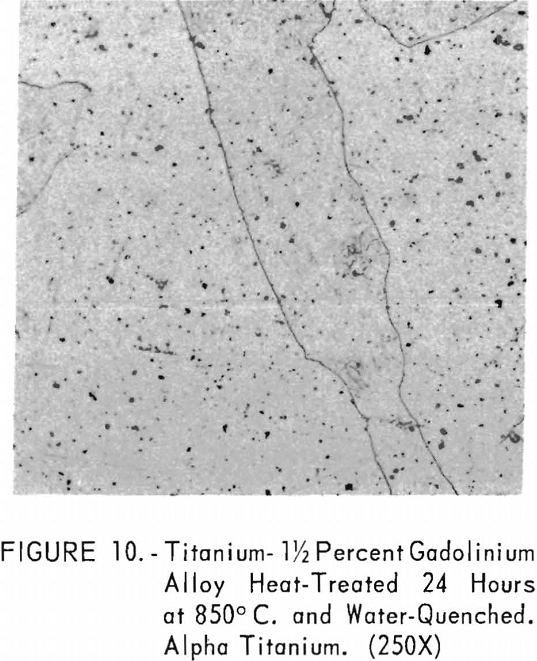
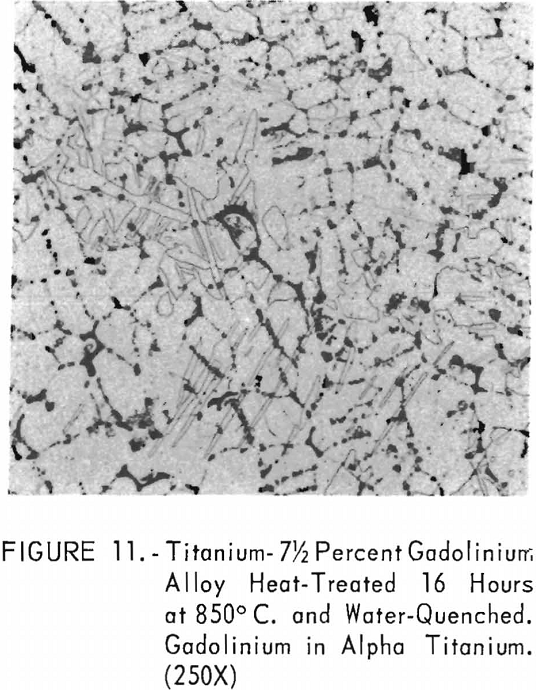
Figures 9 through 11 illustrate the solubility of gadolinium in alpha- titanium at 850° C. The structure of titanium is presented in figure 9. Figure 10 represents the structure of titanium with 1-½ pet. gadolinium added. Both of these structures may be entirely alpha-titanium; however, etch pits and impurities are apparent. In figure 11, the gadolinium content is 7-½ pct., and definite evidence of the gadolinium phase is observed.
Observations to establish solid solubility limits in the titanium-rich region were made on specimens that were not etched. Since these specimens were polished on water-lubricated wheels, the gadolinium phase appeared dark without etching. Under these conditions, the first appearance of the gadolinium was more readily detected.
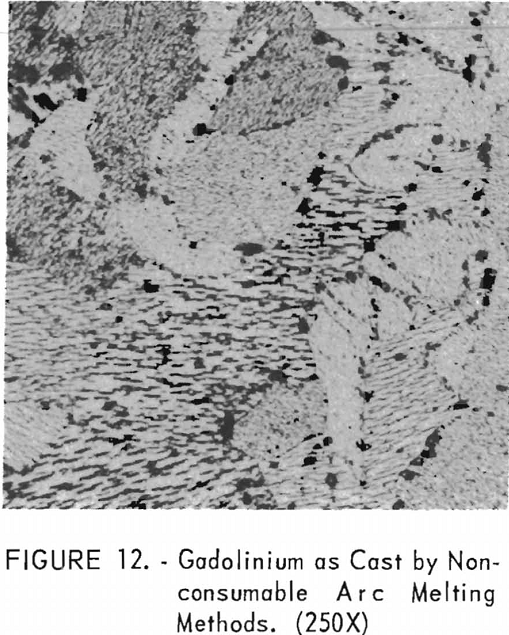
Figure 12 represents the structure of gadolinium in the as-cast condition. A definite grain-orientation pattern is apparent as well as some impurities.
All alloys that contain gadolinium as a second phase reacted extensively with etching reagents. Gadolinium-rich alloys even showed some reaction with water. On the basis of such a limited examination, the investigators believe that alloys of this system will exhibit poor corrosion resistance to water and water solutions.
