Table of Contents
The mineral dressing research here reported is part of a continuing program by the Bureau of Mines to develop new or improved concentration processes for oxidized or partly oxidized lead and zinc ores. A sample of low-grade sulfide-oxide, lead-zinc ore from Jasper County., Mo., containing galena, cerussite, pyromorphite, hemimorphite, smithsonite, and sphalerite was concentrated by froth flotation and tabling to a grade of 66 percent lead. Because of the presence of a high content of slime-size values, the overall recovery of lead was limited to 64 percent at this grade. Tests to recover the zinc by froth flotation and by gravity concentration did not give satisfactory results.
Since its establishment, the Rolla Metallurgy Research Center has conducted studies of base metals as a part of its overall program. This research has been, and is, directed to beneficiating complex and submarginal ores, to retreating rejects from commercial installations, and to developing fundamental data.
Typical of the complex ores within the scope of this program are the partly oxidized lead-zinc ores of the Tri-State district of southwest Missouri. Information supplied by the Mining Branch of this station led to the conclusion that the Crown Point mine in the NE1/4NE1/4 sec. 10, T. 27 N., R. 32 W., near Duenweg, Mo., is representative of such deposits in the Tri-State district. Accordingly, this deposit was sampled by the Mining Branch, and the composite sample served as the ore for this investigation.
Description of Samples
Two samples were taken for this investigation. The original was a composite of 12 equal-weight portions from the stripped area of the Crown Point mine. The second was a similar composite from the same location, Petrographic and chemical analysis proved the two samples to be nearly identical in both composition and character, and they were considered as one in this work.
Petrographic examination showed that the sample contained chert, quartz, shale, tallow-clay, limestone, and an unidentified iron-phosphate mineral. Lead-zinc minerals present were small amounts of galena, cerussite, pyromorphite, hemimorphite, smithsonite, and a trace of sphalerite. Table 1 shows distribution of the lead-zinc minerals. In this report the term oxide lead refers collectively to both cerussite and pyromorphite, and the term oxide zinc to smithsonite and hemimorphite. Qualitative tests showed that the tallow clay contained a small amount of lead and an appreciable amount of zinc.
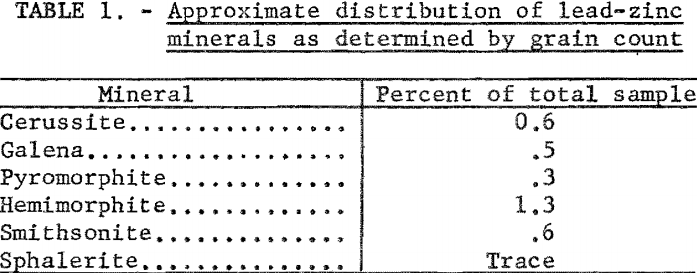
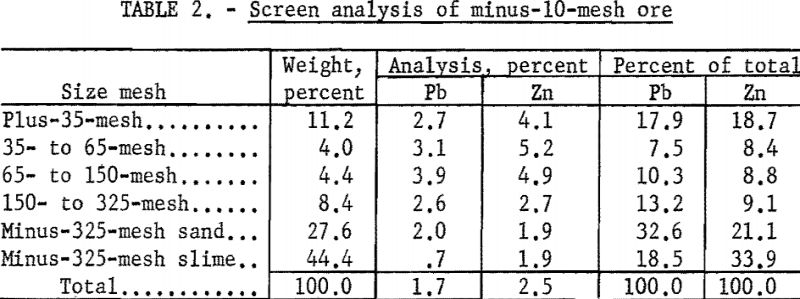
Visual examination of the crude ore showed the galena to be present as crystals and cleavage fragments ranging in size up to one-half inch, and cerussite and smithsonite in smaller aggregates and masses. Other lead-zinc minerals were present in much finer sizes. The larger fragments of galena evidenced much surface oxidation, and both the galena and cerussite were very friable, with a pronounced tendency to form slimes during comminution. In the screen analysis of minus-10-mesh ore (table 2), it will be noted that at even the relatively coarse grind of 10-mesh, 51.1 percent of the lead and 55.0 percent of the zinc were contained in the minus-325-mesh fraction.
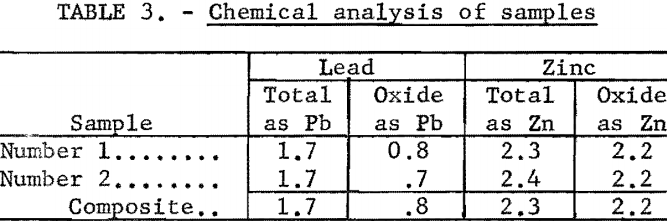
Chemical analyses revealed that the samples were low-grade with an average content of 1.7 percent Pb and 2.3 percent Zn. The lead content was divided about evenly between sulfide and oxidized minerals, but most of the zinc was oxidized. This may be noted from table 3.
Beneficiation Research
The occurrence of galena is widespread, and methods for its concentration are well known. Cerussite, a secondary lead mineral, is less common, but methods for its recovery also have been developed. The third lead mineral in the Crown Point ore, pyromorphite, may be recovered by mineral dressing methods, but a recent Bureau of Mines report noted that this mineral is not amenable to flotation in the presence of clay slimes. Of the zinc minerals present, sphalerite is concentrated readily by either gravity processes or flotation, and smithsonite has been floated successfully by a method developed by Bunge and co-workers. Data are limited on the recovery of hemimorphite. As most of the zinc in this ore is very fine hemimorphite (table 1) associated with tallow-clay, there is little hope for its successful recovery. This conclusion is based upon the results of preliminary tests, and the experimental work described in this report deals mainly with the recovery of the three lead minerals.
Froth Flotation
The high percentage of lead and zinc in the minus-325-mesh fraction, together with the floatability of galena and cerussite, indicated froth flotation as the logical starting point for this investigation. Preliminary studies included an investigation of xanthates and other conventional collectors for both sulfide and oxide minerals as applied to deslimed and undeslimed ores. These studies showed that minus-200-mesh and 15 to 20 percent solids were the most desirable grain size and pulp density for floating undeslimed pulp.
Deslimed pulp
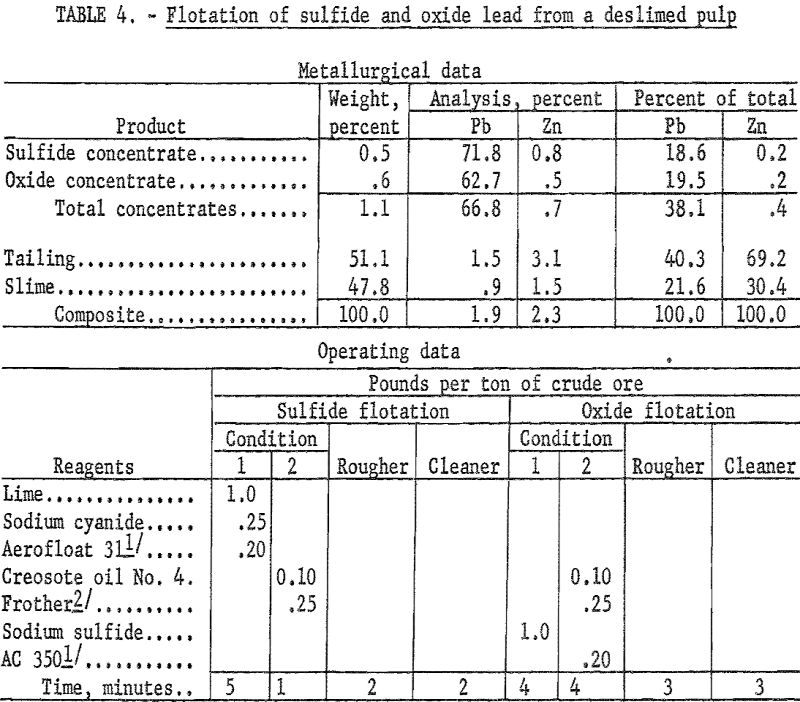
Because of the very large amount of clay slime in this ore the initial flotation tests were made on deslimed samples. Desliming was accomplished both by hydrocycloning and decantation. In each instance the sand fraction was floated for recovery of sulfide and oxide lead using Aerofloat 31 and potassium amyl xanthate as collector for galena and cerussite, respectively.
The combined sulfide-oxide concentrate from a sample deslimed by hydrocycloning (table 4) recovered 38.1 percent of the total lead in a concentrate containing 66.8 percent Pb and 0.7 percent Zn.
Recoveries from flotation of deslimed material ranged from 38 to 41 percent regardless of the desliming method used, but grade was higher in the rougher concentrates from samples deslimed by hydrocycloning. Desliming by either method resulted in heavy losses of both lead and zinc to the slime fraction.
Undeslimed Pulp
To minimize the heavy loss of both lead and zinc to the slimes, flotation research was extended to the uncommon reagents in search of a collector for lead minerals that would be effective in the presence of clay slimes. Among the numerous organic compounds investigated the toluenethiols were especially interesting in that they appeared to be unaffected by the clay slimes in this ore and had characteristics of a collector for both sulfide and oxide lead, thus permitting the simultaneous flotation of galena and cerussite. Of the several toluenethiols tested the one with para orientation of the thiol group was the most effective and was used to a large extent in subsequent work.
A typical test using this reagent as collector on an undeslimed sample was as follows: The sample was ground in the presence of 1.0 pound per ton of sodium silicate to pass a 200-mesh screen, conditioned for 2-minutes with 2.0 pound per ton of sodium sulfide, and floated with 0.67 pound per ton of para-toluenethiol. The rougher froth was cleaned once without additional reagent. The cleaner concentrate resulting from this procedure contained 68.5 percent Pb and 1.0 percent Zn, and represented 58.3 percent and 0.8 percent of the total lead and zinc, respectively. Results of this test are detailed in table 5.
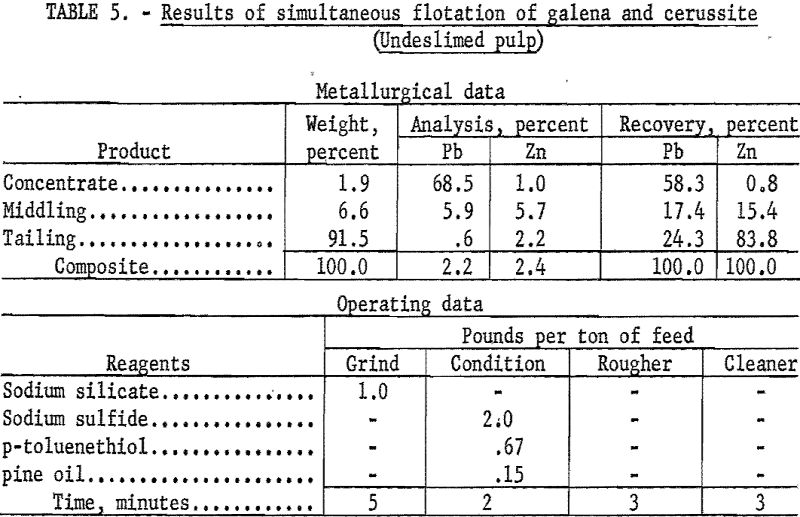
As research progressed it became increasingly evident that flotation alone would not effect a high enough recovery of lead or zinc. A relatively good grade of concentrate was obtainable, but approximately 60 percent of the total lead was the maximum recovery possible by either bulk or selective flotation of lead-bearing minerals.
Flotation Plus Gravity Separation
Having demonstrated that a satisfactory grade of lead concentrate could be obtained by froth flotation, but with too low overall recovery, it was decided to investigate the use of wet-tabling to recover additional lead minerals from the flotation tailing. Petrographic examination of this tailing showed the lead minerals to consist essentially of pyromorphite with scattered grains of cerussite and a trace of galena. The pyromorphite was coarser than either of the latter minerals.
Two general procedures were followed in the flotation-tabling investigation of minus-100-mesh ore. In one the ore was floated directly, using toluenethiol as collector for both galena and cerussite. In the other the ore was deslimed before floating with xanthates as collectors for galena and cerussite. In each case the flotation tailing was wet tabled to concentrate the pyromorphite and to make a rougher zinc concentrate or middling.
As an example of the first procedure (table 6), the wet-ground undeslimed sample was conditioned for 2 minutes with 2.0 pounds of sodium sulfide and floated with 0.67 pound of para-toluenethiol per ton of ore. Flotation was complete in 3 minutes. The rougher concentrate recovered 56.9 percent of the total lead at a grade of 67.5 percent Pb and 0.8 percent Zn. Tabling the flotation tailing recovered an additional 6.8 percent of the total lead at a grade of 60.9 percent Pb and 3.8 percent Zn for a combined recovery of 63.7 percent of the lead in a concentrate containing 66.7 percent Pb and 1.1 percent Zn.
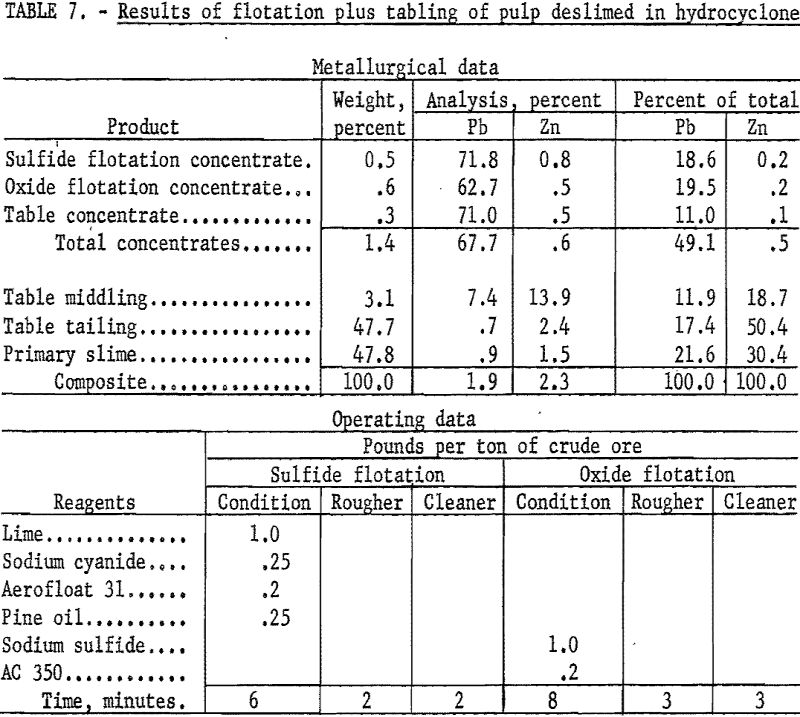
As an example of the second procedure (table 7), the sample was wet-ground with 1.0 pound of sodium silicate per ton of ore and deslimed in a bench scale hydrocyclone. The sand fraction was conditioned with lime and sodium cyanide to depress zinc minerals and floated with Aerofloat 31 to collect the galena. In a second stage the cerussite was conditioned with sodium sulfide and floated with potassium amyl xanthate.
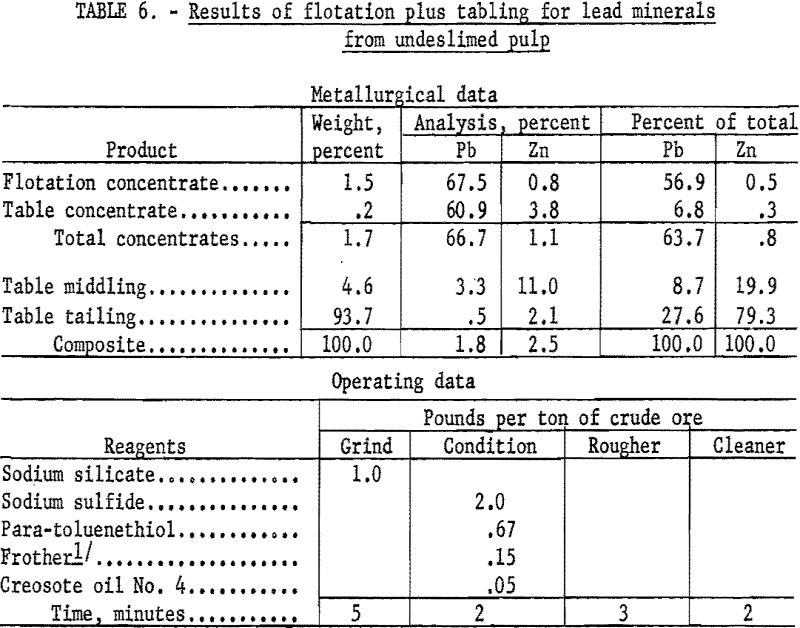
The galena concentrate recovered 18.6 percent of the total lead at a grade of 71.8 percent Pb and 0.8 percent Zn while the cerussite concentrate recovered 19.5 percent at a grade of 62.7 percent Pb and 0.5 percent Zn. Tabling of flotation rejects recovered an additional 11.0 percent of the total lead at a grade of 71.0 percent Pb and 0.5 percent Zn for a combined recovery of 49.1 percent of the total lead in a concentrate containing 67.7 percent Pb and 0.6 percent Zn. Additional lead would be recovered by recirculating the table middlings which contain 11.9 percent of the total lead at a grade of 7.4 percent Pb.
Heavy-Media Separation and Tabling
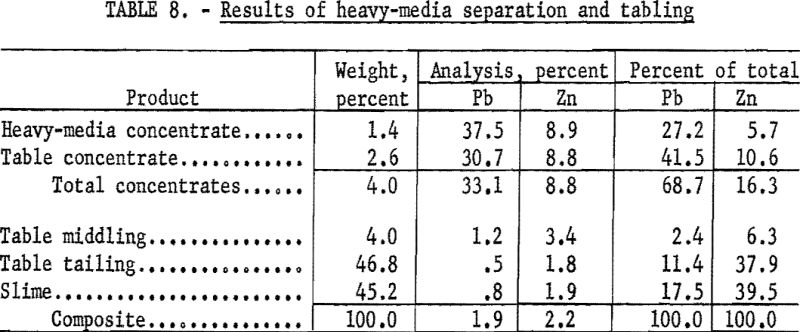
A major difficulty in mineral dressing and one leading to much loss of valuable minerals is the formation of slimes during comminution. This problem was quite serious with the Crown Point ore in which both galena and cerussite were very friable. When the sample was crushed to minus-10-mesh, 51.1 percent of the total lead reported in the minus-325-mesh fraction. This suggested the desirability of recovering these two minerals at near their maximum size, which by visual examination was near ½-inch. This relatively large size restricted choice of methods to jigging or heavy-media separation. Each was investigated, but as the latter proved to be somewhat superior, it was chosen for separating the minus-½-inch, plus-10-mesh fraction of the ore with tabling for the minus-10-mesh fraction. Both methods require blunging to free the desired minerals from the very tenacious tallow-clay.
The plus-4- and plus-10-mesh fractions were treated separately with ferrosilicon heavy medium at 2.85 gravity. Float from the plus-4-mesh fraction was crushed to pass minus-4 and added to the original plus-10 before separation of the latter. All minus-10-mesh material was classified and tabled.
Results of this test (table 8) showed 27.2 percent of the total lead in the heavy-media concentrate and 41.5 percent in the table concentrate at grades of 37.5 and 30.7 percent Pb respectively. This represented an overall recovery of 68.7 percent of the total lead at a grade of 33.1 percent Pb and 8.8 percent Zn. The concentrates were heavily contaminated with an unidentified iron-phosphate mineral.
Flotation of Oxidized Zinc
Recovery of the oxidized zinc minerals contained in the rejects from both flotation and gravity concentration of lead was sought by means of froth flotation, using amines and fatty acids as collectors for the zinc.
In a typical test on the tailing from lead flotation (table 9) the pulp was conditioned in two stages with 16.0 pounds of sodium sulfide and floated with 0.5 pound of coco-amine acetate per ton of ore. This two-stage operation gave a rougher concentrate containing 61.9 percent of the zinc in the feed at a grade of 14.7 percent Zn. This recovery represented 36.6 percent of the total zinc in the crude ore. Stage additions of the reagents in smaller quantities did not improve recovery or grade. Flotation of zinc in the rejects from jig and table concentration of lead resulted in equal recovery but somewhat lower grade.
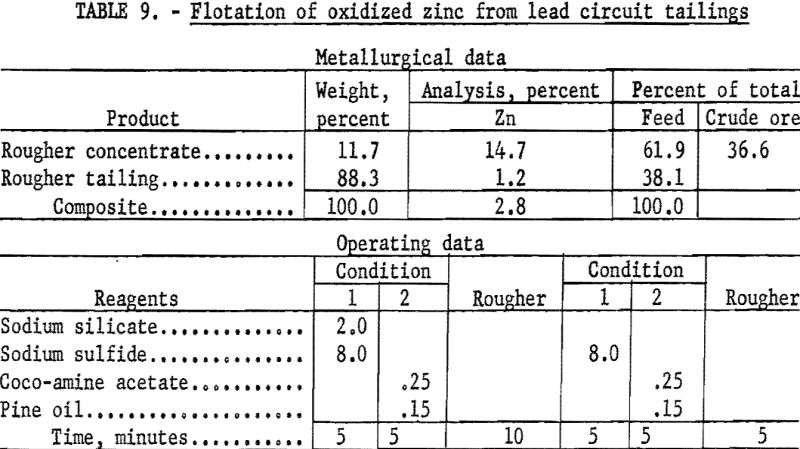
Under the conditions considered in this investigation smithsonite appears to be amenable to flotation, although an excessive amount of sulfidizing agent is required to effect its recovery. Under the same conditions, hemimorphite, which was the major zinc-bearing mineral present, proved unresponsive. A literature search revealed little information on the beneficiation of this silicate mineral. Although it is a minor ore mineral, ranking behind sphalerite and smithsonite in abundance, hemimorphite is of potential worth, and methods for its recovery should be developed through further research.
