Table of Contents
The use of thiourea, (CS(NH2)2 as a complexing/extracting agent for gold and other precious metals has shown promise for implementation in the mining/metals industry. Laboratory testing of this reagent has indicated that a thiourea process for gold extraction has several advantages over conventional processing techniques. The use of cyanide for the extraction of gold from ores and concentrates is the predominant treatment method for primary recovery of this metal throughout the world. Indeed, cyanidation has proven to be very effective and economical for processing many types of gold-bearing materials. However, certain characteristics and disadvantsages of the cyanidation process warrant the investigation and development of alternative gold extractant systems.
The major concerns involving the cyanidation process are the health and environmental problems associated with it. These include the possible formation of deadly hydrogen cyanide gas, the ingestion or absorption through the skin of cyanide salts, and the formation of the free forms of cyanide (HCN and CN-) in effluent waters in concentrations toxic to aquatic life. In light of increasing environmental awareness and responsibility, it is possible that continued stiffening of environmental and health standards will occur. Certainly, increased monitoring of air and water quality can be expected, while some states, such as California, may possibly ban new cyanide processing altogether.
Thiourea is not restricted by the same toxicity factors as cyanide. Thiourea toxicity data indicate high threshold limit values for mammals and a lethal dose of 10 g/kg for humans. On this basis, thiourea is to be considered much safer than cyanide. Indeed, the low pH required in a thiourea process would probably be of more concern. However, it must be mentioned that thiourea has been shown to be a carcinogen to rats and possibly to trout. Even so, thiourea has been used for years in the treatment of thyroid diseases in humans and at this time is considered to be non-carcinogenic to humans.
Another aspect of cyanidation which can be a problem is the somewhat slow kinetics of dissolution of gold by the cyanide species. This can especially be a problem in vat and heap leaching operations which process ores containing fairly large-grained gold particles.
The kinetics of thiourea dissolution of gold have been studied extensively. Initial interest in thiourea as an extractant/complexing agent for gold dates back to a 1941 paper by Plaskin and Kozhukhova. Interest was renewed by these researchers in 1960, and since then several investigators have studied the gold-thiourea system. Several of these investigators studied the rate of dissolution of pure gold in acidic thiourea solutions. Under proper conditions of acid and added oxidant, a thiourea medium has been shown to dissolve the gold as much as twelve times faster than a cyanide medium. The acids studied included sulfuric, hydrochloric, and nitric. The various oxidants examined were ferric ion, hydrogen peroxide, sodium peroxide, formamidine disulfide, dissolved oxygen, ozone, and potassium permanganate. The fastest leaching system has been generally concluded to be the ferric ion-sulfate media system, although chloride media results are very similar. Rates are dependent on thiourea and oxidant concentrations, and appear to be controlled partially by chemical phenomena and partially by transport of oxidant species to the gold surface. A transition from diffusion control to kinetic control takes place at higher stirring speeds. These factors are similar to characteristics of cyanide dissolution of gold.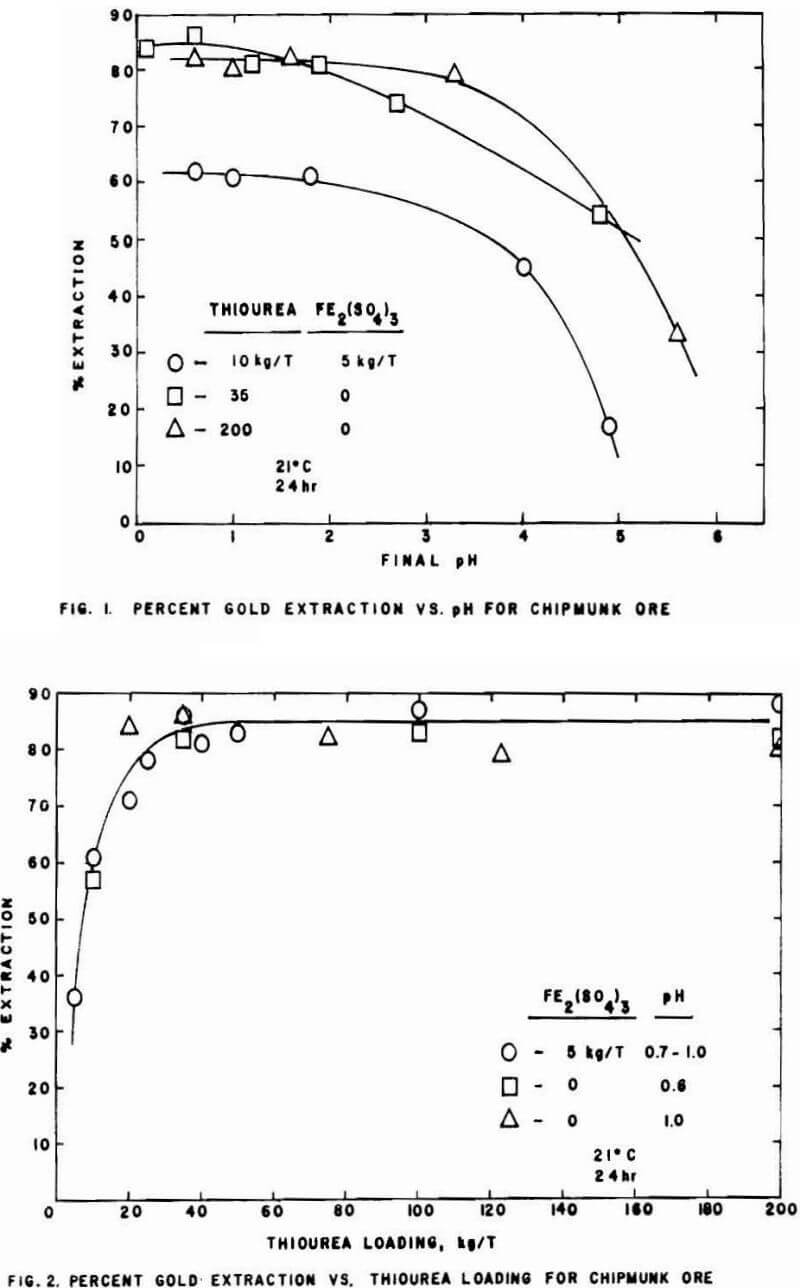
With the exception of one study, investigations on actual ground gold ores comparing cyanidation leaching rates to those of acidic thiourea solutions supported the results of the work on pure gold. However, the increase in leaching rate was not as large as for pure gold samples. Other studies have been conducted on actual ores and/or concentrates with recoveries of greater than 90% claimed using leaching times of eight hours or less.
A third well-known disadvantage of cyanidation is the inhibition of the dissolution of gold caused by certain metal ions and other species. These agents form from dissolution of constituents present in the ore. Such elements as copper and zinc tend to form ionic complexes with cyanide species, thus reducing the availability of cyanide ion for gold dissolution, as well as increasing cyanide consumption. These metal-cyanide species may also indirectly inhibit the gold dissolution reactions. Other elements, such as arsenic and antimony, react with cyanide solutions in such a way as to reduce the availability of dissolved oxygen necessary for the dissolution of gold.
Thiourea leaching has been reported to be affected to a much lesser degree by the presence of some of these cyanidation inhibitors. In fact, one study suggests that copper, antimony, and arsenic minerals, and activated carbon actually increase the effectiveness of thiourea processing of gold. However, other investigators studying thiourea in nitric and hydrochloric acid media report a decrease in solubility in the presence of zinc, copper, and lead salts due to complex formation of these metals with thiourea. Iron minerals present may serve as a partial supply of ferric ion oxidant for the thiourea-gold interaction. All in all, it has been concluded that thiourea has an advantage over cyanidation in the presence of these species.
The major disadvantage of the thiourea process is reported to be the higher cost due to high thiourea and acid consumptions. Excessive thiourea consumption is partially due to the oxidation-degradation of thiourea by ferric iron. Other factors which contribute to thiourea degradation are temperature and complex formation with other metals. It has been shown that thiourea consumption can be reduced by an acid pre-leach to remove impurities.
Given the reported advantages of a thiourea process, it was decided to undertake groundwork experimentation on leaching of a Carlin-type ore. The parameters studied include thiourea concentration, oxidant requirements, pH, leaching time, and leaching temperature. Optimizations of overall gold extraction and thiourea usage were the goals of the experiments.
Adsorption Gold Thiourea & Cation Exchange Resin
Thiourea, is not restricted by the same toxicity factors as cyanide. Toxicity data on thiourea indicate that it has a high threshold limit for mammals and a lethal dose of 10 g/kg for humans. Thiourea has been used for years in the treatment of thyroid diseases in humans and is currently considered non-carcinogenic to humans. However, some studies have shown thiourea to be carcinogenic to rats and trout.
Another disadvantage of cyanidation is the slower kinetics of dissolution of gold species by cyanide. Theoretical considerations indicate that this disadvantage cannot be overcome easily without considerable increases in consumption of reagents and operation costs. The kinetics of thiourea dissolution of gold is several times faster than those of cyanide.
Yet another disadvantage of cyanidation is the inhibition of the dissolution of gold caused by the presence of some ionic species. Species such as Cu, Fe, Ni, Co, Zn, Cd, As, Sb tend to form ionic complexes with cyanide and reduce the availability of cyanide for gold dissolution. The presence of these species affects thiourea leach process to a much lesser degree.
The main disadvantage of the thiourea process is the higher cost of the reagent and the consumption. In spite of this disadvantage, the thiourea process can be used for special materials where the grade will warrant the higher cost of the overall process. The thiourea process has the potential application in the treatment of refractory sulfide and carbonaceous gold ores. These ores are currently pre-treated by roasting, pressure oxidation or bioxidation before cyanidation for gold recovery. These pretreatment techniques all result in the production of acid solutions that require neutralization prior to cyanide leach. Use of acidic thiourea instead of cyanide would allow for immediate leaching of gold without neutralization and result in considerable savings through the elimination of the neutralization step.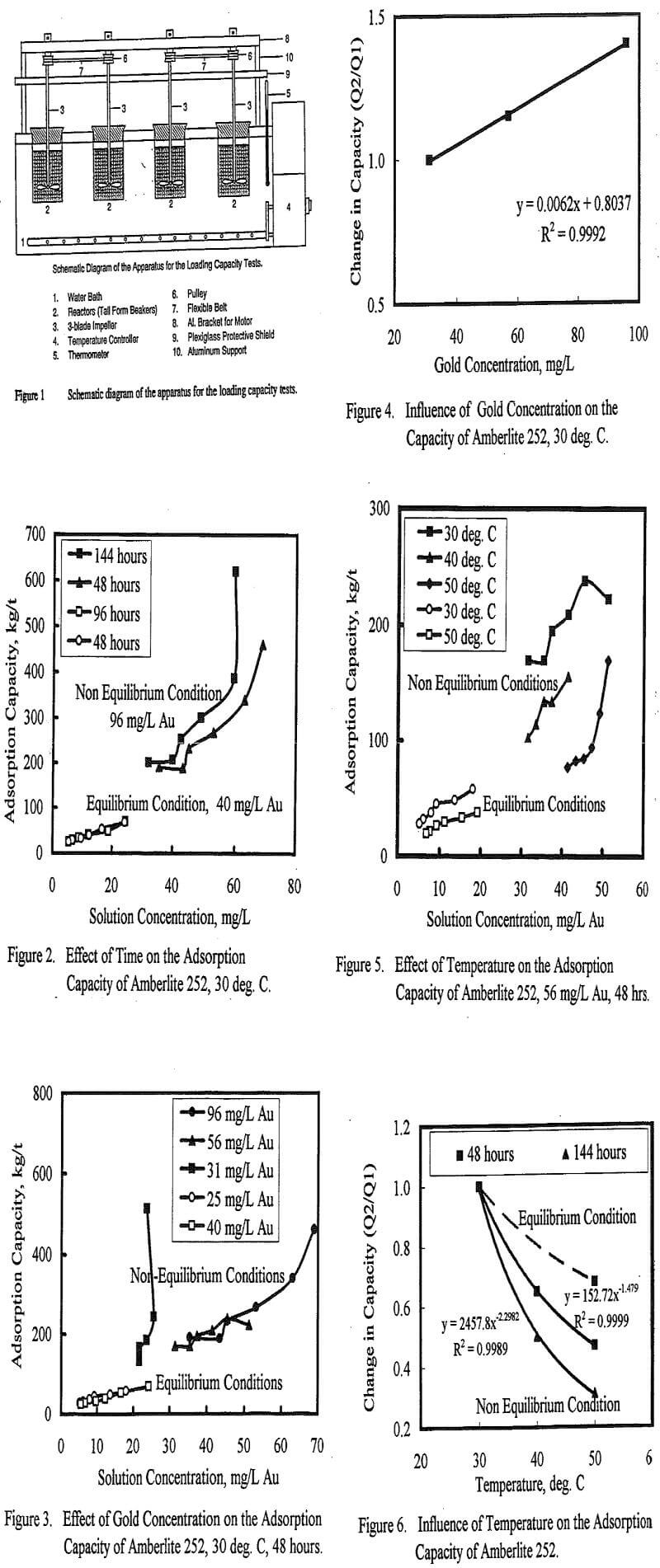
For the thiourea process to compete industrially with cyanidation, it is essential to develop efficient process(es) to recover the gold from solution. Research on the leaching of gold by thiourea has been reported extensively in the literature, but research on the recovery of gold from leach solutions has not been well documented. Some methods for recovering gold-thiourea complex reported in the current literature include electrochemical, activated carbon adsorption, ion-exchange adsorption, precipitation by metal powders and reduction by sulfur dioxide gas. A review of the current literature reveals that there is no clear consensus among researchers concerning the methods for the recovery of gold from thiourea solutions. It was also obvious from the literature that the maximum loading capacity of ion exchange resins for gold-thiourea complex was not higher than 100 kg/t.
Examination of the loaded resin by scanning electron microscope, revealed deposits of elemental gold on the surface of the resin. The presence of elemental gold on the surface of the resin was unexpected and could not be explained at the time of the study. However, other researchers have since reported the presence of elemental gold on the surface of carbon after the latter have been used to adsorb gold-thiourea complex. The presence of elemental gold on the surface of adsorbents suggests some reduction mechanism accompanying the adsorption of gold-thiourea complex.
The proposed mechanism of adsorption of gold-thiourea complex consists of an ion exchange between the cationic gold complex and the positive functional groups on the resin. This adsorbed gold complex is later reduced to elemental gold on the surface of the resin.
The dissolution of gold by thiourea involves the formation of a single cationic species of gold by thiourea in an acidic medium according to the reaction
Au0 + 2CS(NH2)2 = Au[CS(NH2)2]2+ + e-…………………………………………….[1]
During the ion exchange process this single cationic gold species (gold-thiourea complex) is exchanged for the cationic groups on the resin. The general ion exchange reaction is represented as:
RN.H++Au[CS(NH2)2]2+ ↔ RN.AU[CS(NH2)2]2+ + H+………………………………….[2]
In theory this reaction can be reversed and driven in the opposite direction with acids to elute the gold from the loaded resin. However, in practice, strong acid cation exchange resins adsorb the gold-thiourea complex so strongly that complete elution of gold from the resins has been difficult even with high concentration of acids. The present study was not designed to evaluate the reversal of the equilibrium reaction. All the tests were performed to evaluate the forward reaction involving the exchange of the gold-thiourea complex for the cationic groups on the resin.
The adsorption capacity of the resin was calculated from the concentration of gold in the initial and final solution as mg of gold adsorbed per g of resin used. These values were converted to meq Au per g of resin or kg Au per tonne of resin. The influence of the following variables on the adsorption capacity was evaluated:
Thiourea Concentration

The effect of thiourea concentration on the adsorption capacity of Amberlite 252 for gold-thiourea complex was also evaluated. Three levels of initial thiourea concentration were evaluated, 0.025M, 0.125M and 0.25 M. The adsorption capacity data are tabulated in Table 7 and the isotherms are shown in Figure 8. The adsorption capacity of Amberlite 252 from a 0.025M thiourea solution in 48 hours ranged from 132 to 541 kg/t and decreased with increasing thiourea concentration. The adsorption capacity decreased by about 65% when the initial thourea concentration was increased from 0.025M to 0.125M. Further increase in the initial thiourea concentration to 0.25M resulted in the decrease in the adsorption capacity value by 85%. This relation between thiourea concentration and adsprption capacity may be useful for eluting the loaded resin.
The correlation between the change in adsorption capacity and thiourea concentration was represented by equation [10] and is shown in Figure 9:
ΔQ = – 0.376 ln[TU] – 0.4……………………………………………..[10]
where [TU] is the initial thiourea concentration in moles/liter (M), ΔQ(Q2/Q1) is the change in average adsorption capacity, Q2 is the average capacity from a solution with any given initial thiourea concentration and Q1 is the average capacity from a 0.025M thiourea solution. We can predict from this equation that increasing the thiourea concentration to 0.3M will achieve a reduction of 95%. Although we do not have experimental data to support this, our previous studies with the gold-bromine system revealed that elution of gold-bromide from loaded resins was possible with more than 0.5M thiourea solution.
Summary and Conclusion
This study has demonstrated that adsorption of gold from acidic thiourea solution by a cationic ion exchange resin is feasible. Capacity values greater than 100 kg/t were achieved within 48 hours of contact time and values greater than 400 kg/t were achieved after 144 hours of contact. Equilibrium adsorption could not be attained within the contact times investigated due to the proposed reduction reaction accompanying the ion exchange process. These high adsorption capacity values and the phenomenon of gold reduction on the resin were observed under conditions of high level of resin utilization. The apparent saturation capacity of the resin under these non equilibrium conditions was several times higher than the ion exchange capacity due to the contribution from the reduction reaction. The reduction reaction resulting in the deposition of gold on the resin is possible through electron exchange reaction between the resin matrix and the gold-thiourea complex. Evidence of the deposition of gold on the resin was revealed by scanning electron microscopy with EDAX. Adsorption isotherms generated from the raw data revealed that several levels of apparent saturation capacity can occur on each isotherm.
Under non equilibrium conditions, the adsorption capacity increased with increasing gold concentration and decreased with increasing temperature and thiourea concentration. The influence of these variables on the adsorption capacity was not consistent with equilibrium adsorption behavior. The influence of temperature and thiourea concentration on the adsorption capacity may be useful for elution of the loaded resin. The correlation between adsorption capacity, temperature and thiourea concentration predicted that loaded resins could be eluted with a high concentration of thiourea and at high temperatures (≥ 0.3 M thiourea at 120° C).
Equilibrium adsorption was attained when the level of resin utilization was low. The equilibrium adsorption capacity was lower than the values obtained under the non equilibrium conditions (103±10 kg/t versus 400 kg/t). Under equilibrium conditions, temperature and gold concentration only slightly affected the adsorption capacity. This was consistent with ion exchange adsorption equilibria. Scanning electron microscopy with EDAX did not reveal any deposition of gold under equilibrium adsorption conditions.
Thiourea Consumption during Gold Leaching
Gold is dissolved in an acidic thiourea solution according to the following anodic reaction:
Au + 2CS(NH2)2 = Au(CS(NH2)2)2+ + e-……………………………………………………(1)
Ferric sulfate is a preferred oxidant because of its mild oxidation potential and fast kinetics for gold dissolution. However, it is important to note that the standard potential of the ferric/ferrous ion pair is 771mV and that of thiourea/formamidine disulfide is 420mV. It is not surprising that an excessive amount of ferric iron would cause thiourea decomposition. The first oxidation product of thiourea using ferric iron was examined by HPLC and UV/visible spectroscopy and found to be formamidine disulfide (Bukka et al., 1992). The formamidine disulfide can further decompose, which is believed to occur via the following reaction sequence:
Fe+3 + CS(NH2)2 = Fe+2 + ½NH(NH2)C-S-S-C(NH2)NH + H +…………………………………(2)
NH(NH2)C-S-S-C(NH2)NH → CS(NH2)2 + Sulfinic Compound……………………………………(3)
Sulfinic Compound → CN·NH2 + S°…………………………………………………………………………….(4)
The formamidine disulfide further decomposes or is oxidized irreversibly, which will cause a net consumption of thiourea. The reaction between thiourea and ferric iron appears to be a major cause of thiourea decomposition.
A general rate expression for thiourea decomposition according to equation (2) can be written as:
-d[Tu]/dt = -d[Fe+3]/dt = k[Tu]n[Fe+3]m…………………………………………………………(5)
where [Tu] is the concentration of residual thiourea, [Fe+3] is the concentration of residual ferric iron, t is time, k is the rate constant, n and m are the reaction orders with respect to the concentrations of thiourea and total ferric iron.
The rate of thiourea decomposition is shown to be equivalent to the rate of ferric iron consumption from the correlation presented in Fig. 1.
The effect of thiourea and ferric iron concentrations on the decomposition rate of thiourea and reduction rate of ferric iron to ferrous iron was measured and the results are plotted in Fig.2 and Fig. 3. In these experiments, both the thiourea and ferric iron concentrations varied with time so that the reaction orders were determined using the initial rates of thiourea decomposition and ferric iron consumption. The values of n and m were found to be 1 by plotting of log{- d[Tu]/dt}t=0 and log [-d[Fe+3/dt]=0 derived from the data presented in Fig.2 and Fig.3 versus log[Tu]t=0 and log[Fe+3]t=0 respectively as shown in Fig. 4 and Fig. 5.
Reaction (2) is rather slow in these solutions and was found to be first order with respect to both total ferric iron concentration and thiourea concentration. It is believed that this situation would favor gold leaching from ores or concentrates because a higher potential could be maintained and the rate of thiourea decomposition would be slow. However, few papers have been published regarding the characteristics of this reaction with respect to the presence of different types of minerals. In this regard, the effect of three minerals, quartz, hematite and pyrite has been examined.
Comparison of these mineral effects on the rate of the thiourea decomposition reaction is shown by the plots of residual thiourea concentration(g/l) versus time in Figures 6, 7, 8 and 9 for a reference solution with an initial concentration of thiourea 4.0g/l, total ferric iron concentration 3.0g/l, and pH 1.5 at a temperature 18°C. The total solution volume was 1000ml. It can be seen that quartz does not have any effect on the rate of decomposition. However, hematite and pyrite significantly enhance the reaction, and the pyrite effect is more significant than that of hematite. For example, when 5 grams of pyrite is added, the thiourea is completely decomposition is not only sensitive to mineral composition, but also to the mineral particle sizes, which indicates that the hematite and pyrite effect is associated with a surface reaction which facilitates the decomposition of thiourea.

