In a paper, entitled A Uniform Method for the Assay of Copper Material for Gold and Silver, A. R. Ledoux invited the assayers of this country to contribute to a symposium, in which the results of their assays of given samples of copper-matte and metallic copper would be made comparable. This symposium appeared about one year thereafter; and through it and its discussion, first became generally known the fact that the gold- yield by the all-fire method of assaying is usually higher than by the so-called combination (wet and dry) method, in which nitric acid is employed as a solvent for the copper. In the publication alluded to, the supposed cause of gold-losses in the latter method was declared to be the solvent action of copper nitrate, or of the reduction-products of the nitric acid, upon the gold. Up to a very recent date, gold has been held to be insoluble in pure nitric acid. (The solvent action of any chlorine present in the nitric acid must here properly be disregarded as belonging in the realm of carelessness.)
Among later allusions to this topic, W. R. Van Liew ascribes the solution of the gold in the combination-assay entirely to the action of nitrous acid at the high reaction-temperature of copper and nitric acid; F. B. Flinn believes the low result in gold to be due exclusively to mechanical losses—finely-divided gold penetrating through the filter-paper—but presents no experimental data confirmative of this belief; and 0. Pufahl attributes the solubility of the gold in some coppers to the presence of selenium—a view which appears to be shared by many assayers. There seems, however, to be no foundation in fact for such an assumption. We do know that gold is dissolved by concentrated selenic acid when heated. When copper with minute selenium-content is dissolved in nitric acid, selenious acid is formed, which is highly diluted, and has, to our knowledge, no action whatever on the gold. Recently, F. P. Dewey has convincingly demonstrated that pure gold dissolves in small quantity, on long-continued boiling, in pure concentrated nitric acid.
Before discussing experiments and theories on this subject, it may be well to state the chemical composition of the various auriferous copper-bullions, and briefly to describe their treatment in commercial sampling and assaying, since in this field lies the practical importance of a thorough knowledge of the problems involved.
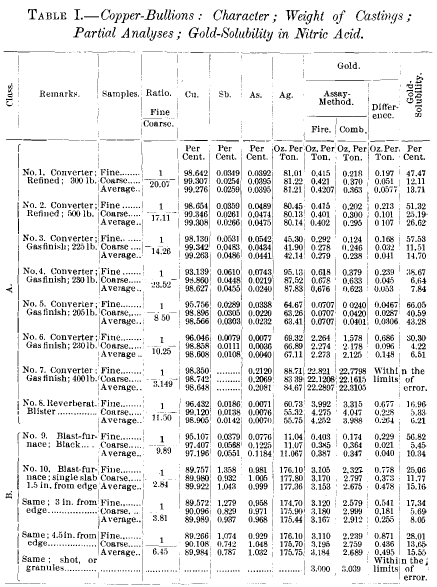
In shape, copper-bullion castings are generally known as pigs (ingots), and slabs (plates), the former being thick and the latter thin, as compared to their other dimensions. The first form favors and the second minimizes so-called segregation. The weight of the castings ranges approximately between 200 and 600 lb. They are sampled by drilling through their entire thickness—the holes being placed according to regular templates; and the drillings are brought to the proper fineness by grinding. In several of our large copper-refineries the grinding process is carried to a point where the whole sample will pass a 16- or even a 20-mesh screen. In my own practice these samples are separated into coarse and fine parts by means of a 40-mesh screen. It is self-evident that the fine part will contain all such extraneous matter as the oxides, slags, mold-wash and incidental dirt, while the coarse part consists of clean metallic (alloy) particles. Table I. shows differences in metal-values of the fine and coarse parts which demonstrate the necessity of separate assays of the two and the averaging of the results according to the ratio of the parts; or else of making combined assays of the fine and coarse, weighed and mixed in their proper ratio. Some assayers believe they can accomplish this object equally well by reducing the mixed sample to approximately the desired weight by some mechanical cutting- down device.
As regards the methods of assaying the copper-bullions for gold, little need be said of the all-fire method. It assumes that all the copper is eliminated by scorification and cupellation. In these two operations, gold-losses occur by slagging and absorption; but, if these be determined and accounted for, operations by this method under varied conditions yield concordant results, which are the most accurate attainable. The nitric acid combination-method presents a very different case. Here, variation in heat-conditions, or in the dilution of the acid used in dissolving the copper, causes varying quantities of the gold to be dissolved, and therefore lost.
In the tests embodied in this paper, a uniform procedure was employed. To 1 assay-ton of the copper was added 190 cc. of distilled water with 90 cc. of pure nitric acid (sp. gr. 1.42); and after the violence of the reaction had subsided, the beaker was placed on a steam-plate, at 160° F., where it was left until the red fumes had been expelled. No chloride was used to precipitate the silver; but the gold was gathered on a triple S. & S., No. 597, 12.5-cm. filter covered with 2 g. of test-lead; and, after incineration of the filter and the addition of 20 g. of test-lead, a sufficient quantity of silver, in the form of nitrate crystals, was added. That part of the combination-method designated as “dry” needs no description, since what has been said of the all-fire method holds true here, namely, that when the so-called fire-losses are determined and accounted for, variations in this part of the method do not alter the results.
When, therefore, we have determined the amount of gold by the all-fire method and the amount by the combination-method, the difference should be equal to the gold-loss by solution in the latter method. (The suggestion that this difference may involve other factors will be discussed hereafter.) All the results for gold given in this paper are averages, generally of five assays, often of more. The accuracy of the all-fire method is, almost without exception, within 0.03 oz. per ton, while the combination-method sometimes shows on the higher-grade bullions differences as high as 0.2 oz. per ton. The measurement by the ounce per ton has been employed throughout for silver and gold, not because this is the commercial custom, but because it is much more convenient for comparison than the extended decimal fractions expressing percentages. In carrying out the various assays and analyses, I was assisted by K. W. McComas and Albert Ferrell.
From Table I. it will be seen that, on account of the admixture of extraneous impurities, already referred to, the copper- content in the fines is always lower than in the coarse, while in many cases (class A) the fines show an enrichment in other elements.
The examples of copper-bullions in Table I. have been divided into two classes, A and B. In general, it may be remarked that to class A belong all those of a copper-tenor above 97 per cent., and to class B those of a copper-tenor below that figure. In the castings of class A, the precious metals and other elements are found concentrated towards the freezing- center of the castings, while in class B they are found richer towards the surfaces. None of the elements in question, however, show the same degree of heterogeneity, so that, in some instances, the two classes overlap each other. Viewed as solutions, class A must be considered as copper subsaturated above its freezing-point (hypo-eutectic); while in class B the copper is saturated above its freezing-point (hyper-eutectic). In class A the copper is the element freezing first; in class B the other elements freeze first.
Looking again at the composition of the samples, fine and coarse, it will be noted that under class A the fines, generally, are higher in foreign elements than the coarse; and it is consistent to conclude that the fines (exclusive of extraneous matter), in their greater portion, are derived from the later-freezing parts of the bullion castings. The gold in the fines shows a markedly greater solubility in nitric acid than the gold in the coarse, which, as will be seen later, is in conformity with the supposition that the fines are derived from the last and most slowly-cooled portions of the castings. The fines in class B show a smaller amount of impurities than the coarse, thereby also proving their origin from the last-freezing portions of the bullion. In them, moreover, the gold-solubility is much greater than in the coarse.
The samples of bullions, as regards their metallurgical origin, are as follows: Nos. 1 and 2, converted copper, furnace-refined; Nos. 3,4,5, 6, and 7, converted copper, gas-finished; No. 8, reverberatory blister-copper; Nos. 9 and 10, blast-furnace black copper.
My first serious attention to the solubility of gold in nitric acid was drawn by the peculiar facts connected with bullions Nos. 1 and 2, Table I. They were derived from the same source and treated identically in the process of refining; often coming from the same furnaces, which statement finds corroboration in the results of the partial analyses. Both were quenched (pickled) as soon as solidified. Two parallel series of gold- assays were made for many millions of pounds of material; one series being by the all-fire method, the other by the combination-method. The results of these two series of assays were, that for bullion No. 1, the gold-yield by the combination- method showed a deficit of 13.71 per cent., as compared with the all-fire method, while for No. 2 the corresponding figure was 26.62 per cent.—both, it may be repeated, under identical conditions of assaying. Since, as already remarked, all the assays were corrected for the so-called fire-losses, it followed that the results were to be so interpreted that the figures 13.71 per cent, and 26.62 per cent, denoted the solubility-losses of the gold in the two respective bullions—in one nearly twice as great as in the other. Although these two bullions were the same in origin, composition, and metallurgical treatment, there remained one evident difference in the castings, as to size and weight; No. 1 weighing about 300 lb., and No. 2 over 500 lb. This fact implied a difference in the rate of cooling, and led at once to the conclusion that gold contained in slowly-cooled copper is more soluble in nitric acid than that in rapidly-chilled copper. That the gold-solubility was not influenced by any mechanical differences in the two samples is evidenced by the fact that the ratios of fine and coarse in both are very nearly alike, while the size of the coarse particles in both samples is the same, yet the solubility of the gold in the coarse is for No. 1 only one-half of that in No. 2.
If, then, the conclusion reached be true, that the solubility in nitric acid of the gold in the same bullion or alloy is subject to great variation according to the rate of cooling, it should be possible to reduce this solubility to a minimum by the instantaneous chilling of the molten bullion. Granulation, or shotting, by running a stream of the molten bullion into cold water, suggested itself, and advantage was taken of a blast¬furnace charge of copper, for an experiment, to compare the behavior of the gold in a shot-sample with that in a drill- sample from a large and slowly-cooled slab. The results of the tests are incorporated in Table I., bullion No. 10. They demonstrated at once the correctness of the hypothesis; none of the gold in the granules or shot being soluble, while the absolute amount of gold from the slowly-cooled slab dissolved by nitric acid was greater than in any other of the samples of copper-bullion or alloys that have been tested.
Thus far, the conclusions reached were based on the analytical methods; it now appeared desirable to proceed to the synthetical.
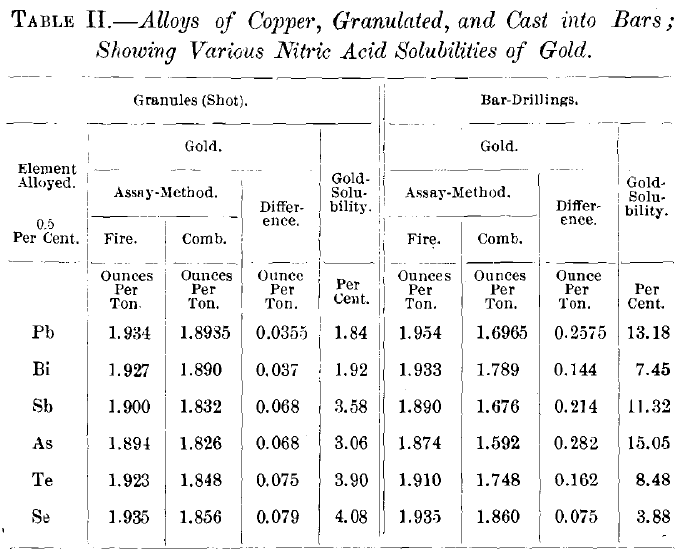
Table II. shows the solubility of the gold in shot and drillings of a series of alloys, each of which was prepared by adding
0. 5 per cent, of a foreign element and an amount of gold equaling approximately 2 oz. per ton to commercial electrolytic copper. The alloy was thoroughly fused and mixed under a charcoal cover in a crucible. About 3,000 g. of the alloy was cast into a heated mold to form a small ingot; the remainder being granulated. Each ingot was drilled in the same manner for a sample, while the granules could directly be used for analysis. It would be venturing too much to claim for the solubility-figures in Table II. that they are the true relative expressions of the influence of each of the given elements on the solubility of the gold contained in the ingots, for there was, probably, a wide variation between them in the conditions of cooling and freezing. In the process of granulating it is far more likely that those conditions are nearly alike in all cases, and that here the effects of the alloyed elements on the gold-solubility are shown in their proper relative degrees. It seems to be demonstrated that the quenching effect diminishes from the metallic towards the metalloidal end of the series, reaching zero with selenium.
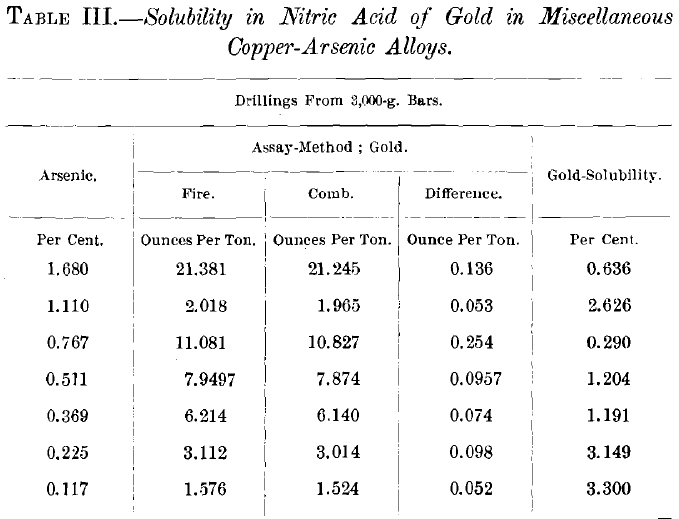
The largest quantity of soluble gold thus far found is 0.495 oz. per ton, or 15.55 per cent, of the total gold in this special case, or 0.0017 per cent, of the total alloy-which is a very small proportion. It became of interest, therefore, to determine if this figure could be materially enlarged by the increase of either the gold- or the impurity-content of the copper-alloy.
Table III. shows the negative results on alloys with varying tenors of gold and arsenic. In these cases small ingots were cast in heated molds, which might not preclude too rapid cooling; therefore, tests of the slowest possible cooling were instituted, one of which is exemplified in Table IV., with negative results. This shows also how the great gold-solubility in the fine portion of a sample is reduced by remelting and granulation.
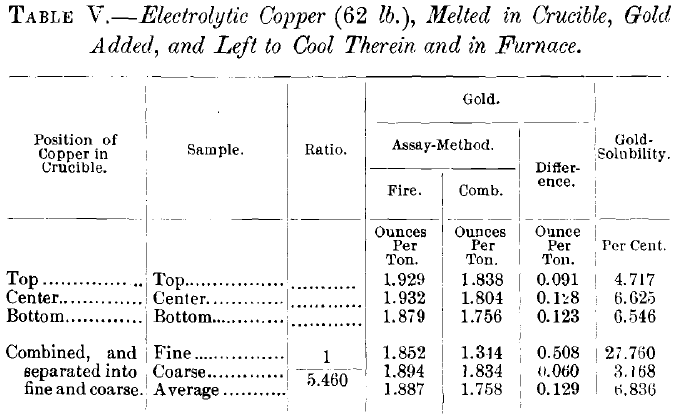
Since neither gold-increase nor impurity-increase appeared to augment the quantity of soluble gold, it became a question what influence pure copper would have in the solubility of a relatively small gold-content. Table V. gives the results of a test with the purest commercial electrolytic copper to which gold was added by melting. It shows that the gold is as soluble in this as in many of the ordinary bullions; but, since the purest commercial electrolytic copper still contains as much, or more, of arsenic and antimony, per cent., as there is soluble gold in most of the bullions, and absolutely pure copper is not available in quantity, the effect of the latter remains unknown.
The tests on the solubility of gold in nitric acid were also extended to a number of lead-bullions; all with results that closely approached the negative. Table VI. gives those obtained from a comparatively large and slowly-cooled casting.
Reference has already been made in the beginning to the bearing of this subject upon the practical sampling and assaying of auriferous copper-bullious. Ignorance in this field has, in the past, led to some acrimonious controversies, the possibilities of which may be understood most readily through an illustration: A furnace-charge of molten copper would be sampled by shotting (properly done); then several samples by drilling would be taken from slabs, varying from a thin sample plate to the largest-sized commercial slab. The gold-assays on all the samples would now, by one party, be made by the all-fire method, with concordant results, and with the verdict that all the methods of sampling are correct. A second party, believing the combination-method reliable, would, by its use, find concordant results between the shot-sample and the thin-plate sample, while with the other samples the results would show an increasing deficit, reaching its maximum, perhaps 30 per cent., with the sample from the largest slab. This party’s pronouncement would be : shot and thin-plate sampling correct; samples from large casting absolutely wrong.
It may be observed in passing that the accuracy in the gold- assay has been multiplied many times during the past decade through the remarkable improvement in the construction of gold-balances.
At the present day there probably exist but few contracts in the copper-world which permit the employment of the nitric acid combination-method for the assay of gold in copper-bullions, although this method may still be pronounced quite permissible for quick routine-work, when properly modified, and, especially, when applied to quenched or otherwise rapidly- cooled samples.
Summary:
I. Observed Facts—Gold contained in copper-alloys, or bullions, is soluble in nitric acid in varying degrees; the solubility diminishing with the increased rate of cooling of the castings, and reaching a minimum when the molten bullion is quenched in cold water. In nearly all of the quenched samples the gold, determined by the combination-method of assaying, still shows a deficit as compared with the amount found by the all-fire assay ; the maximum of this deficit being approximately 2 per cent.
The highest amount of soluble gold was found to be 0.0017 per cent, of the total bullion; no increase in gold- or impurity- content was able to augment that amount.
A definite relation between gold-solubility and impurity- content of the bullions has not been established; the former being more dependent on the rate of cooling than on the quality or quantity of impurity. This proposition is demonstrated by the fact that the gold-solubility in all samples is greatest in the fine portions, which in classes A and B are the most slowly- cooled parts (this conclusion being deduced from the chemical composition). In class A the fine portion is more impure than the coarse; in class B this relation is reversed.
The observations by Dewey of the solubility of pure gold in boiling concentrated nitric acid, and those of Van Liew for avoiding the solution of gold in copper-bullion by dissolving the latter in very dilute nitric acid under ice-cooling, are not controverted.
II. Hypothetical Conclusions.—The smallness of the absolute amount of soluble gold contained in bullions makes it doubtful whether the actual condition of this gold can, at present, be determined by any of the known methods, chemical or physical.
From what is known of the action of strong nitric acid, or other chemicals, on pure or uncombined gold, it would follow that these would not discriminate between such gold obtained in slowly-cooled and in quenched bullions. It is, furthermore, not probable that nitric acid, of the dilution used in dissolving these bullions, and in the presence of a great excess of metallic copper, would attack uncombined gold. The difference in the properties of the gold in slowly-cooled and in quenched bullions will, therefore, rationally be sought in a change of chemical conditions, or of the chemical nature of the alloy itself, by the heat-treatment. It seems justifiable to assume that a portion of the gold in slowly-cooled bullion combines with some, or all, of the accompanying elements, forming compounds soluble in nitric acid; that the reaction takes place through a narrow range of temperature above the freezing-point, and that it is a slow one as compared to the speed of cooling; that at higher temperature the gold-compounds dissociate; and that quenching of the bullions at those temperatures leaves the gold in the uncombined state, and, therefore, insoluble in the nitric acid used to dissolve the bullion. Since neither gold- nor impurity- increase augments the amount of soluble gold beyond a low maximum, it would appear that the soluble gold-compounds are formed only in very dilute solutions, or under low osmotic pressure.
The fact that with the seleniferous alloy quenching has no reducing effect on the gold-solubility, may be explained by the supposition that probably the gold-selenium compound forms at higher temperatures than the others, or that it has not dissociated at the temperatures obtaining in the furnace or crucible at which quenching is performed.
The 2 per cent, or less of gold-deficit, as found by the combination-assay in quenched bullions, may be due to a residual soluble gold-compound, formed on account of the impossibility of instantaneous chilling by quenching. Should this explanation be unacceptable, then it could be conceded that this loss is due to all other causes that have been suggested for the loss of uncombined gold.
Phenomena of similar nature to those suggested in the foregoing have been well established by scientific investigation. C. T. Heycock and F. H. Neville have demonstrated that gold in metallic solution forms chemical compounds with metals, as for instance, tin as a solvent, gold and cadmium combine in the alloy to form in succession the following compounds : Au4Cd4, Au5Cd5, Au9Cd9. One of the instances best known to metallurgists of a chemical compound being formed at a certain temperature and being dissociated at higher ones, is the case of iron carbide (cementite) in steel. The carbide is formed when steel is slowly cooled, and its formation is prevented when the steel is quenched above the dissociation-temperature; chemical conditions are here changed by heat-treatment. In other respects there is much dissimilarity between the carbide in steel and the hypothetical gold-compounds in copper-bullion. The carbide is formed well below the freezing-point of the steel; it is not attacked by dilute sulphuric and hydrochloric acids, while in quenched steel the dissociation-products readily combine ; the iron to form a salt, the carbon a hydride. In the quenching process the heat of combination and crystallization remained latent and this becomes instrumental in forming the compound with the acids. An equally well-known case in which, by heat-treatment, a physical change only is wrought, is that of the quenched blast-furnace slags. When these are permitted to cool slowly they attain the crystalline state and become insoluble in acids, while when the molten slags are run into cold water they remain amorphous; the heat of crystallization remains latent, and they are readily attacked by the acids.
NOTE.- The amount of copper taken in the analysis for antimony and arsenic was from 10 to 25g., depending on the grade of the metal.

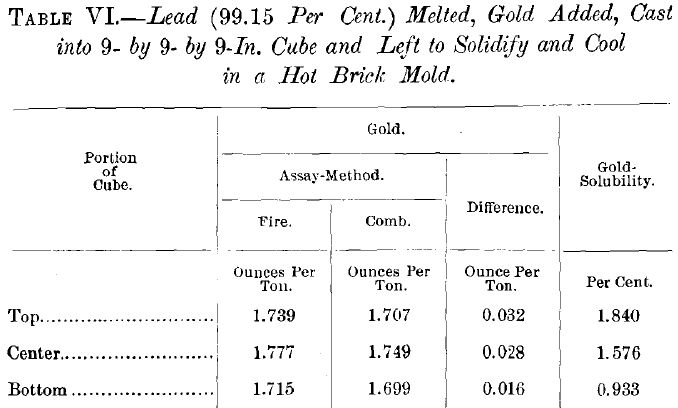
The Solubility in Nitric Acid of Gold Contained in Certain Copper-Alloys (Copper-Bullions). BY EDWARD KELLER, PERTH AMBOY, N. J.
